「Estradiol」の版間の差分
細 (エストロゲンへの転送ページ) タグ: 新規リダイレクト |
細 (1版 をインポートしました) |
||
| 1行目: | 1行目: | ||
# | {{About|estradiol as a hormone|its use as a medication|Estradiol (medication)}} | ||
{{Use dmy dates|date=August 2018}} | |||
{{Chembox | |||
<!-- Images --> | |||
| ImageFile1 = Estradiol.svg | |||
| ImageClass1 = skin-invert | |||
| ImageSize1 = 225px | |||
| ImageAlt1 = The chemical structure of estradiol. | |||
| ImageFile2 = Estradiol 3D ball.png | |||
| ImageSize2 = 225px | |||
| ImageAlt2 = A ball-and-stick model of estradiol. | |||
<!-- Names --> | |||
| pronounce = {{IPAc-en|ˌ|ɛ|s|t|r|ə|ˈ|d|aɪ|oʊ|l}} {{respell|ES|trə|DY|ohl}}<ref name="FordRoach2013" /><ref name="HochadelMosby2015" /> | |||
| IUPACName = Estra-1,3,5(10)-triene-3,17β-diol | |||
| SystematicName = (1''S'',3a''S'',3b''R'',9b''S'',11a''S'')-11a-Methyl-2,3,3a,3b,4,5,9b,10,11,11a-decahydro-1''H''-cyclopenta[''a'']phenanthrene-1,7-diol | |||
| OtherNames = Oestradiol; E2; 17β-Estradiol; 17β-Oestradiol | |||
| Watchedfields = changed | |||
| verifiedrevid = 488623959 | |||
<!-- Sections --> | |||
| Section1 = {{Chembox Identifiers | |||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| CASNo = 50-28-2 | |||
| ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| ChEBI = 16469 | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 135 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 5554 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB00783 | |||
| EINECS = 200-023-8 | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D00105 | |||
| PubChem = 5757 | |||
| SMILES = C[C@]12CC[C@@H]3c4ccc(cc4CC[C@H]3[C@@H]1CC[C@@H]2O)O | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1 | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = VOXZDWNPVJITMN-ZBRFXRBCSA-N | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = 4TI98Z838E | |||
}} | |||
| Section2 = {{Chembox Properties | |||
| C=18 | H=24 | O=2 | |||
| MolarMass = 272.38 g/mol | |||
| Appearance = | |||
| Density = | |||
| MeltingPt = | |||
| BoilingPt = | |||
| Solubility = | |||
| MagSus = -186.6·10<sup>−6</sup> cm<sup>3</sup>/mol | |||
}} | |||
| Section3 = {{Chembox Hazards | |||
| MainHazards = | |||
| FlashPt = | |||
| AutoignitionPt = | |||
}} | |||
| Section6 = {{Chembox Pharmacology | |||
| ATCvet = | |||
| ATCCode_prefix = G03 | |||
| ATCCode_suffix = CA03 | |||
| ATC_Supplemental = | |||
| Licence_EU=yes | |||
| AdminRoutes = [[Oral administration|Oral]], [[sublingual administration|sublingual]], [[intranasal administration|intranasal]], [[topical medication|topical]]/[[transdermal]], [[intravaginal administration|vaginal]], [[intramuscular injection|intramuscular]] or [[subcutaneous injection|subcutaneous]] (as an [[estradiol ester|ester]]), [[subdermal implant]] | |||
| Bioavail = Oral: <5%<ref name="StanczykArcher2013" /> | |||
| Excretion = [[Urine]]: 54%<ref name="StanczykArcher2013" /><br />[[Feces]]: 6%<ref name="StanczykArcher2013" /> | |||
| HalfLife = Oral: 13–20 hours<ref name="StanczykArcher2013" /><br />Sublingual: 8–18 hours<ref name="PriceBlauer1997" /><br />Topical (gel): 36.5 hours<ref name="NauntonAl Hadithy2006" /> | |||
| Metabolism = [[Liver]] (via [[hydroxylation]], [[sulfation]], [[glucuronidation]]) | |||
| ProteinBound = ~98%:<ref name="StanczykArcher2013" /><ref name="FalconeHurd2007" /><br />• [[Human serum albumin|Albumin]]: 60%<br />• {{abbr|SHBG|sex hormone-binding globulin}}: 38%<br />• Free: 2% | |||
}} | |||
}} | |||
'''Estradiol''' ('''E2'''), also called '''oestrogen''', '''oestradiol''', is an [[estrogen]] [[steroid hormone]] and the major [[female]] [[sex hormone]]. It is involved in the regulation of female [[reproductive cycle]]s such as [[estrous cycle|estrous]] and [[menstrual cycle]]s. Estradiol is responsible for the development of female [[secondary sexual characteristic]]s such as the [[breast]]s, [[widening of the hips]] and a [[gynoid fat distribution|female pattern of fat distribution]]. It is also important in the development and maintenance of female [[reproductive organ|reproductive tissues]] such as the [[mammary gland]]s, [[uterus]] and [[vagina]] during [[puberty]], [[adulthood]] and [[pregnancy]].<ref name="pmid7083198" /> It also has important effects in many other [[tissue (biology)|tissues]] including [[bone]], [[fat]], [[skin]], [[liver]], and the [[brain]]. | |||
Though estradiol levels in males are much lower than in females, estradiol has important roles in males as well. Apart from humans and other [[mammal]]s, estradiol is also found in most [[vertebrate]]s and [[crustacean]]s, [[insect]]s, [[fish]], and other [[animal]] [[species]].<ref name="Mechoulam_2005" /><ref name="Ozon_1972" /> | |||
Estradiol is produced within the [[ovarian follicle|follicles]] of the [[ovary|ovaries]] and in other tissues including the [[testicle]]s, the [[adrenal gland]]s, fat, [[liver]], the breasts, and the brain. Estradiol is [[biosynthesis|produced in the body]] from [[cholesterol]] through a series of [[chemical reaction|reactions]] and [[chemical intermediate|intermediates]].<ref>Saldanha, Colin J., Luke Remage-Healey, and Barney A. Schlinger. "Synaptocrine signaling: steroid synthesis and action at the synapse." Endocrine reviews 32.4 (2011): 532–549.</ref> The major [[metabolic pathway|pathway]] involves the formation of [[androstenedione]], which is then converted by [[aromatase]] into [[estrone]] and is subsequently converted into estradiol. Alternatively, androstenedione can be converted into [[testosterone]], which can then be converted into estradiol. Upon [[menopause]] in females, production of estrogens by the ovaries stops and estradiol levels decrease to very low levels. | |||
In addition to its role as a natural hormone, estradiol is used as a [[medication]], for instance in [[menopausal hormone therapy]], and [[feminizing hormone therapy]] for [[Trans woman|transgender women]] and other [[genderqueer]] individuals; for information on estradiol as a medication, see the [[estradiol (medication)]] article. | |||
{{TOC limit|3}} | |||
== Biological function == | |||
=== Sexual development === | |||
{{See also|Breast development#Biochemistry}} | |||
The development of [[secondary sex characteristics]] in women is driven by estrogens, to be specific, estradiol.<ref name="McMillanFeigin2006" /><ref name="CraigStitzel2004" /> These changes are initiated at the time of [[puberty]], most are enhanced during the reproductive years, and become less pronounced with declining estradiol support after [[menopause]]. Thus, estradiol produces [[breast development]], and is responsible for changes in the [[body shape]], affecting bones, joints, and [[fat deposition]].<ref name="McMillanFeigin2006" /><ref name="CraigStitzel2004" /> In females, estradiol induces breast development, [[widening of the hips]], a [[gynoid fat distribution|feminine fat distribution]] (with fat deposited particularly in the breasts, hips, thighs, and buttocks), and maturation of the [[vagina]] and [[vulva]], whereas it mediates the [[pubertal growth spurt]] (indirectly via increased [[growth hormone]] secretion)<ref name="Preedy2011" /> and [[epiphyseal closure]] (thereby limiting [[human height|final height]]) in both sexes.<ref name="McMillanFeigin2006" /><ref name="CraigStitzel2004" /> | |||
=== Reproduction === | |||
==== Female reproductive system ==== | |||
In the female, estradiol acts as a growth hormone for tissue of the reproductive organs, supporting the lining of the [[vagina]], the cervical glands, the [[endometrium]], and the lining of the fallopian tubes. It enhances growth of the [[myometrium]]. Estradiol appears necessary to maintain [[oocyte]]s in the [[ovary]]. During the [[menstrual cycle]], estradiol produced by the growing follicles triggers, via a positive feedback system, the hypothalamic-pituitary events that lead to the [[luteinizing hormone]] surge, inducing ovulation. In the luteal phase, estradiol, in conjunction with [[progesterone]], prepares the endometrium for [[Implantation (human embryo)|implantation]]. During [[pregnancy]], estradiol increases due to [[placenta]]l production. The effect of estradiol, together with [[estrone]] and [[estriol]], in [[pregnancy]] is less clear. They may promote uterine blood flow, myometrial growth, stimulate breast growth and at term, promote cervical softening and expression of myometrial [[oxytocin]] receptors.{{citation needed|date=February 2013}} In baboons, blocking of estrogen production leads to pregnancy loss, suggesting estradiol has a role in the maintenance of pregnancy. Research is investigating the role of estrogens in the process of initiation of [[Childbirth|labor]]. Actions of estradiol are required before the exposure of progesterone in the luteal phase.{{citation needed|date=February 2013}} | |||
==== Male reproductive system ==== | |||
The effect of estradiol (and estrogens in general) upon male reproduction is complex. Estradiol is produced by action of [[aromatase]] mainly in the [[Leydig cell]]s of the [[mammal]]ian [[testicle|testis]], but also by some [[germ cell]]s and the [[Sertoli cell]]s of immature mammals.<ref>{{cite journal | vauthors = Carreau S, Lambard S, Delalande C, Denis-Galeraud I, Bilinska B, Bourguiba S | title = Aromatase expression and role of estrogens in male gonad : a review | journal = Reproductive Biology and Endocrinology | volume = 1 | pages = 35 | date = April 2003 | pmid = 12747806 | pmc = 155680 | doi = 10.1186/1477-7827-1-35 | doi-access = free }}</ref> It functions (''[[in vitro]]'') to prevent [[apoptosis]] of male [[sperm]] cells.<ref>{{cite journal | vauthors = Pentikäinen V, Erkkilä K, Suomalainen L, Parvinen M, Dunkel L | title = Estradiol acts as a germ cell survival factor in the human testis in vitro | journal = The Journal of Clinical Endocrinology and Metabolism | volume = 85 | issue = 5 | pages = 2057–67 | date = May 2000 | pmid = 10843196 | doi = 10.1210/jcem.85.5.6600 | doi-access = free }}</ref> | |||
While some studies in the early 1990s claimed a connection between globally declining [[sperm count]]s and estrogen exposure in the environment,<ref>{{cite journal | vauthors = Sharpe RM, Skakkebaek NE | title = Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? | journal = Lancet | volume = 341 | issue = 8857 | pages = 1392–5 | date = May 1993 | pmid = 8098802 | doi = 10.1016/0140-6736(93)90953-E | s2cid = 33135527 }}</ref> later studies found no such connection, nor evidence of a general decline in sperm counts.<ref>{{cite journal | vauthors = Handelsman DJ | title = Estrogens and falling sperm counts | journal = Reproduction, Fertility, and Development | volume = 13 | issue = 4 | pages = 317–24 | date = 2001 | pmid = 11800170 | doi=10.1071/rd00103}}</ref><ref>{{cite journal |vauthors=Fisch H, Goldstedin R |title=Environmental estrogens and sperm counts |journal=Pure and Applied Chemistry |date=2003 |volume=75 |issue=11–12 |pages=2181–2193 |url=http://pac.iupac.org/publications/pac/pdf/2003/pdf/7511x2181.pdf |doi=10.1351/pac200375112181 |s2cid=11068097 |access-date=29 December 2015 |archive-date=4 March 2016 |archive-url=https://web.archive.org/web/20160304102628/http://pac.iupac.org/publications/pac/pdf/2003/pdf/7511x2181.pdf |url-status=dead }}</ref> | |||
Suppression of estradiol production in a subpopulation of subfertile men may improve the [[semen]] analysis.<ref>{{cite journal | vauthors = Raman JD, Schlegel PN | title = Aromatase inhibitors for male infertility | journal = The Journal of Urology | volume = 167 | issue = 2 Pt 1 | pages = 624–9 | date = February 2002 | pmid = 11792932 | doi = 10.1016/S0022-5347(01)69099-2 }}</ref> | |||
Males with certain [[sex chromosome]] [[genetic condition]]s, such as [[Klinefelter's syndrome]], will have a higher level of estradiol.<ref>{{cite journal | vauthors = Visootsak J, Graham JM | title = Klinefelter syndrome and other sex chromosomal aneuploidies | journal = Orphanet Journal of Rare Diseases | volume = 1 | issue = 42 | pages = 42 | date = October 2006 | pmid = 17062147 | pmc = 1634840 | doi = 10.1186/1750-1172-1-42 | doi-access = free }}</ref> | |||
=== Skeletal system === | |||
Estradiol has a profound effect on bone. Individuals without it (or other estrogens) will become tall and [[eunuch]]oid, as [[epiphyseal]] closure is delayed or may not take place.<ref>{{cite journal | vauthors = Vanderschueren D, Laurent MR, Claessens F, Gielen E, Lagerquist MK, Vandenput L, Börjesson AE, Ohlsson C | display-authors = 6 | title = Sex steroid actions in male bone | journal = Endocrine Reviews | volume = 35 | issue = 6 | pages = 906–60 | date = December 2014 | pmid = 25202834 | pmc = 4234776 | doi = 10.1210/er.2014-1024 }}</ref> [[Bone density]] is also affected, resulting in early [[osteopenia]] and [[osteoporosis]].<ref>{{cite journal | vauthors = Carani C, Qin K, Simoni M, Faustini-Fustini M, Serpente S, Boyd J, Korach KS, Simpson ER | display-authors = 6 | title = Effect of testosterone and estradiol in a man with aromatase deficiency | journal = The New England Journal of Medicine | volume = 337 | issue = 2 | pages = 91–5 | date = July 1997 | pmid = 9211678 | doi = 10.1056/NEJM199707103370204 | doi-access = free }}</ref> Low levels of estradiol may also predict fractures, with post-menopausal women having the highest incidence of [[bone fracture]].<ref>{{cite journal | vauthors = Bergh C, Wennergren D, Möller M, Brisby H | title = Fracture incidence in adults in relation to age and gender: A study of 27,169 fractures in the Swedish Fracture Register in a well-defined catchment area | journal = PLOS ONE | volume = 15 | issue = 12 | pages = e0244291 | date = 2020-12-21 | pmid = 33347485 | pmc = 7751975 | doi = 10.1371/journal.pone.0244291 | bibcode = 2020PLoSO..1544291B | doi-access = free }}</ref> Women past menopause experience an accelerated loss of bone mass due to a relative estrogen deficiency.<ref>{{cite journal| vauthors = Albright F, Smith PH, Richardson AM |title=Postmenopausal Osteoporosis: Its Clinical Features|journal=[[JAMA (journal)|JAMA]]|author-link1 = Fuller Albright|date=31 May 1941 |volume=116 |issue=22 |pages=2465–2474 |doi=10.1001/jama.1941.02820220007002 }}</ref> | |||
=== Skin health === | |||
The [[estrogen receptor]], as well as the [[progesterone receptor]], have been detected in the [[human skin|skin]], including in [[keratinocyte]]s and [[fibroblast]]s.<ref name="pmid12762829">{{cite journal | vauthors = Raine-Fenning NJ, Brincat MP, Muscat-Baron Y | title = Skin aging and menopause : implications for treatment | journal = American Journal of Clinical Dermatology | volume = 4 | issue = 6 | pages = 371–8 | year = 2003 | pmid = 12762829 | doi = 10.2165/00128071-200304060-00001 | s2cid = 20392538 }}</ref><ref name="pmid16120154">{{cite journal | vauthors = Holzer G, Riegler E, Hönigsmann H, Farokhnia S, Schmidt JB, Schmidt B | s2cid = 6077829 | title = Effects and side-effects of 2% progesterone cream on the skin of peri- and postmenopausal women: results from a double-blind, vehicle-controlled, randomized study | journal = The British Journal of Dermatology | volume = 153 | issue = 3 | pages = 626–34 | date = September 2005 | pmid = 16120154 | doi = 10.1111/j.1365-2133.2005.06685.x }}</ref> At [[menopause]] and thereafter, decreased levels of female [[sex hormone]]s result in [[atrophy]], thinning, and increased [[wrinkling]] of the skin and a reduction in skin [[elasticity (physics)|elasticity]], firmness, and strength.<ref name="pmid12762829" /><ref name="pmid16120154" /> These skin changes constitute an acceleration in [[Human skin#Aging|skin aging]] and are the result of decreased [[collagen]] content, irregularities in the [[morphology (biology)|morphology]] of [[epidermis (skin)|epidermal]] [[skin cell]]s, decreased [[ground substance]] between [[skin fiber]]s, and reduced [[capillary|capillaries]] and [[blood flow]].<ref name="pmid12762829" /><ref name="pmid16120154" /> The skin also becomes more [[dry skin|dry]] during menopause, which is due to reduced skin [[tissue hydration|hydration]] and [[sebum|surface lipids]] (sebum production).<ref name="pmid12762829" /> Along with chronological aging and photoaging, estrogen deficiency in menopause is one of the three main factors that predominantly influences skin aging.<ref name="pmid12762829" /> | |||
[[Hormone replacement therapy]] consisting of systemic treatment with estrogen alone or in combination with a progestogen, has well-documented and considerable beneficial effects on the skin of postmenopausal women.<ref name="pmid12762829" /><ref name="pmid16120154" /> These benefits include increased skin collagen content, skin thickness and elasticity, and skin hydration and surface lipids.<ref name="pmid12762829" /><ref name="pmid16120154" /> Topical estrogen has been found to have similar beneficial effects on the skin.<ref name="pmid12762829" /> In addition, a study has found that topical 2% progesterone cream significantly increases skin elasticity and firmness and observably decreases wrinkles in peri- and postmenopausal women.<ref name="pmid16120154" /> Skin hydration and surface lipids, on the other hand, did not significantly change with topical progesterone.<ref name="pmid16120154" /> These findings suggest that progesterone, like estrogen, also has beneficial effects on the skin, and may be independently protective against skin aging.<ref name="pmid16120154" /> | |||
=== Nervous system === | |||
{{further|Hypothalamic–pituitary–gonadal axis}} | |||
Estrogens can be produced in the [[brain]] from steroid precursors. As [[antioxidants]], they have been found to have [[neuroprotective]] function.<ref>{{cite journal | vauthors = Behl C, Widmann M, Trapp T, Holsboer F | title = 17-beta estradiol protects neurons from oxidative stress-induced cell death in vitro | journal = Biochemical and Biophysical Research Communications | volume = 216 | issue = 2 | pages = 473–82 | date = November 1995 | pmid = 7488136 | doi = 10.1006/bbrc.1995.2647 }}</ref> | |||
The positive and negative [[feedback loop]]s of the [[menstrual cycle]] involve ovarian estradiol as the link to the hypothalamic-pituitary system to regulate [[gonadotropin]]s.<ref>{{cite journal | vauthors = Meethal SV, Liu T, Chan HW, Ginsburg E, Wilson AC, Gray DN, Bowen RL, Vonderhaar BK, Atwood CS | title = Identification of a regulatory loop for the synthesis of neurosteroids: a steroidogenic acute regulatory protein-dependent mechanism involving hypothalamic-pituitary-gonadal axis receptors | journal = Journal of Neurochemistry | volume = 110 | issue = 3 | pages = 1014–27 | date = August 2009 | pmid = 19493163 | pmc = 2789665 | doi = 10.1111/j.1471-4159.2009.06192.x }}</ref> | |||
Estrogen is considered to play a significant role in women's mental health, with links suggested between the hormone level, mood and well-being. Sudden drops or fluctuations in, or long periods of sustained low levels of estrogen may be correlated with significant mood-lowering. Clinical recovery from depression postpartum, perimenopause, and postmenopause was shown to be effective after levels of estrogen were stabilized and/or restored.<ref>{{cite journal | vauthors = Douma SL, Husband C, O'Donnell ME, Barwin BN, Woodend AK | title = Estrogen-related mood disorders: reproductive life cycle factors | journal = Advances in Nursing Science | volume = 28 | issue = 4 | pages = 364–75 | year = 2005 | pmid = 16292022 | doi = 10.1097/00012272-200510000-00008 | s2cid = 9172877 }}</ref><ref>{{cite journal | vauthors = Lasiuk GC, Hegadoren KM | title = The effects of estradiol on central serotonergic systems and its relationship to mood in women | journal = Biological Research for Nursing | volume = 9 | issue = 2 | pages = 147–60 | date = October 2007 | pmid = 17909167 | doi = 10.1177/1099800407305600 | s2cid = 37965502 }}</ref> | |||
The volumes of [[sexual dimorphism|sexually dimorphic]] brain structures in [[transgender women]] were found to change and approximate typical female brain structures when exposed to estrogen concomitantly with androgen deprivation over a period of months,<ref name="eje-utrecht">{{cite journal | vauthors = Hulshoff HE, Cohen-Kettenis PT, Van Haren NE, Peper JS, Brans RG, Cahn W, Schnack HG, Gooren LJ, Kahn RS | date = July 2006 | title = Changing your sex changes your brain: influences of testosterone and estrogen on adult human brain structure | journal = European Journal of Endocrinology | volume = 155 | issue = suppl_1 | pages = 107–114 | doi = 10.1530/eje.1.02248 | doi-access = free }}</ref> suggesting that estrogen and/or androgens have a significant part to play in sex differentiation of the brain, both [[prenatal]]ly and later in life. | |||
There is also evidence the programming of adult male sexual behavior in many vertebrates is largely dependent on estradiol produced during prenatal life and early infancy.<ref>{{cite journal | vauthors = Harding CF | title = Hormonal modulation of singing: hormonal modulation of the songbird brain and singing behavior | journal = Annals of the New York Academy of Sciences | volume = 1016 | pages = 524–39 | date = June 2004 | issue = 1 | pmid = 15313793 | doi = 10.1196/annals.1298.030 | bibcode = 2004NYASA1016..524H | s2cid = 12457330 | url = http://www.annalsnyas.org/content/vol1016/issue1/index.dtl | archive-url = https://web.archive.org/web/20070927225947/http://www.annalsnyas.org/content/vol1016/issue1/index.dtl | url-status = dead | archive-date = 27 September 2007 }}</ref> It is not yet known whether this process plays a significant role in human sexual behavior, although evidence from other mammals tends to indicate a connection.<ref>{{cite journal | vauthors = Simerly RB | title = Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain | journal = Annual Review of Neuroscience | volume = 25 | pages = 507–36 | date = 27 March 2002 | pmid = 12052919 | doi = 10.1146/annurev.neuro.25.112701.142745 | url = http://www.healthsystem.virginia.edu/internet/neuroscience/BehavioralNeuroscience/Simerley-EFR-1-4.pdf | access-date = 7 March 2007 | archive-date = 1 October 2008 | archive-url = https://web.archive.org/web/20081001223447/http://www.healthsystem.virginia.edu/internet/neuroscience/BehavioralNeuroscience/Simerley-EFR-1-4.pdf | url-status = dead }}</ref> | |||
Estrogen has been found to increase the [[secretion]] of oxytocin and to increase the [[gene expression|expression]] of its [[receptor (biochemistry)|receptor]], the [[oxytocin receptor]], in the [[brain]].<ref name="GoldsteinMeston2005">{{cite book | vauthors = Goldstein I, Meston CM, Davis S, Traish A | title = Women's Sexual Function and Dysfunction: Study, Diagnosis and Treatment|url=https://books.google.com/books?id=3J7TnwpbZQwC&pg=PA205|date=17 November 2005|publisher=CRC Press|isbn=978-1-84214-263-9|pages=205–}}</ref> In women, a single dose of estradiol has been found to be sufficient to increase circulating oxytocin concentrations.<ref name="Acevedo-RodriguezMani2015">{{cite journal | vauthors = Acevedo-Rodriguez A, Mani SK, Handa RJ | title = Oxytocin and Estrogen Receptor β in the Brain: An Overview | journal = Frontiers in Endocrinology | volume = 6 | pages = 160 | year = 2015 | pmid = 26528239 | pmc = 4606117 | doi = 10.3389/fendo.2015.00160 | doi-access = free }}</ref> | |||
=== Gynecological cancers === | |||
Estradiol has been tied to the development and progression of cancers such as breast cancer, ovarian cancer and endometrial cancer. Estradiol affects target tissues mainly by interacting with two [[nuclear receptor]]s called [[estrogen receptor α]] (ERα) and [[estrogen receptor β]] (ERβ).<ref name=flav>{{cite journal | vauthors = Bulzomi P, Bolli A, Galluzzo P, Leone S, Acconcia F, Marino M | title = Naringenin and 17beta-estradiol coadministration prevents hormone-induced human cancer cell growth | journal = IUBMB Life | volume = 62 | issue = 1 | pages = 51–60 | date = January 2010 | pmid = 19960539 | doi = 10.1002/iub.279 | s2cid = 7903757 | doi-access = free }}</ref><ref name=pome>{{cite journal | vauthors = Sreeja S, Santhosh Kumar TR, Lakshmi BS, Sreeja S | title = Pomegranate extract demonstrate a selective estrogen receptor modulator profile in human tumor cell lines and in vivo models of estrogen deprivation | journal = The Journal of Nutritional Biochemistry | volume = 23 | issue = 7 | pages = 725–32 | date = July 2012 | pmid = 21839626 | doi = 10.1016/j.jnutbio.2011.03.015 }}</ref> One of the functions of these estrogen receptors is the modulation of [[gene expression]]. Once estradiol binds to the ERs, the receptor complexes then bind to specific [[DNA sequences]], possibly causing damage to the DNA and an increase in cell division and [[DNA replication]]. [[Eukaryotic cell]]s respond to damaged DNA by stimulating or impairing G1, S, or G2 phases of the cell cycle to initiate [[DNA repair]]. As a result, cellular transformation and cancer cell proliferation occurs.<ref name=estrogen>{{cite journal | vauthors = Thomas CG, Strom A, Lindberg K, Gustafsson JA | title = Estrogen receptor beta decreases survival of p53-defective cancer cells after DNA damage by impairing G₂/M checkpoint signaling | journal = Breast Cancer Research and Treatment | volume = 127 | issue = 2 | pages = 417–27 | date = June 2011 | pmid = 20623183 | doi = 10.1007/s10549-010-1011-z | s2cid = 6752694 }}</ref> | |||
=== Cardiovascular system === | |||
Estrogen affects certain [[blood vessel]]s. Improvement in arterial blood flow has been demonstrated in [[coronary artery|coronary arteries]].<ref name="pmid7788912">{{cite journal | vauthors = Collins P, Rosano GM, Sarrel PM, Ulrich L, Adamopoulos S, Beale CM, McNeill JG, Poole-Wilson PA | title = 17 beta-Estradiol attenuates acetylcholine-induced coronary arterial constriction in women but not men with coronary heart disease | journal = Circulation | volume = 92 | issue = 1 | pages = 24–30 | date = July 1995 | pmid = 7788912 | doi = 10.1161/01.CIR.92.1.24 }}</ref> 17-beta-estradiol (E2) is considered the most potent estrogen found in humans. E2 influences vascular function, apoptosis, and damage during cardiac ischemia and reperfusion. E2 can protect the heart and individual cardiac myocytes from injuries related to ischemia. After a heart attack or long periods of hypertension, E2 inhibits the adverse effects of pathologic remodeling of the heart.<ref>{{cite journal |last1=Knowlton |first1=A.A. |last2=Lee |first2=A.R. |title=Estrogen and the cardiovascular system |journal=Pharmacology & Therapeutics |date=July 2012 |volume=135 |issue=1 |pages=54–70 |doi=10.1016/j.pharmthera.2012.03.007 |pmid=22484805 |pmc=5688223 }}</ref> | |||
During [[pregnancy]], high levels of estrogens, namely estradiol, increase [[coagulation]] and the risk of [[venous thromboembolism]]. | |||
{{Venous thromboembolism incidence during pregnancy and the postpartum period}} | |||
=== Other functions === | |||
Estradiol has complex effects on the [[liver]]. It affects the production of multiple [[protein]]s, including [[lipoprotein]]s, binding proteins, and proteins responsible for [[blood clotting]].{{citation needed|date=February 2013}} In high amounts, estradiol can lead to [[cholestasis]], for instance [[intrahepatic cholestasis of pregnancy|cholestasis of pregnancy]]. | |||
Certain gynecological conditions are dependent on estrogen, such as [[endometriosis]], [[leiomyoma]]ta uteri, and [[uterine bleeding]].{{Citation needed|date=February 2013}} | |||
== Biological activity == | |||
{{See also|Pharmacodynamics of estradiol#Mechanism of action}} | |||
Estradiol acts primarily as an [[agonist]] of the [[estrogen receptor]] (ER), a [[nuclear receptor|nuclear]] [[steroid hormone receptor]]. There are two subtypes of the ER, [[estrogen receptor alpha|ERα]] and [[estrogen receptor beta|ERβ]], and estradiol potently binds to and activates both of these receptors. The result of ER activation is a modulation of [[gene transcription]] and [[gene expression|expression]] in ER-expressing [[Cell (biology)|cells]], which is the predominant mechanism by which estradiol mediates its biological effects in the body. Estradiol also acts as an agonist of [[membrane estrogen receptor]]s (mERs), such as [[GPER]] (GPR30), a recently discovered non-nuclear receptor for estradiol, via which it can mediate a variety of rapid, non-[[genomic]] effects.<ref name="pmid24530924">{{cite journal | vauthors = Prossnitz ER, Barton M | title = Estrogen biology: new insights into GPER function and clinical opportunities | journal = Molecular and Cellular Endocrinology | volume = 389 | issue = 1–2 | pages = 71–83 | date = May 2014 | pmid = 24530924 | pmc = 4040308 | doi = 10.1016/j.mce.2014.02.002 }}</ref> Unlike the case of the ER, GPER appears to be [[binding selectivity|selective]] for estradiol, and shows very low [[affinity (pharmacology)|affinities]] for other endogenous estrogens, such as estrone and [[estriol]].<ref name="pmid17222505">{{cite journal | vauthors = Prossnitz ER, Arterburn JB, Sklar LA | title = GPR30: A G protein-coupled receptor for estrogen | journal = Mol. Cell. Endocrinol. | volume = 265–266 | pages = 138–42 | year = 2007 | pmid = 17222505 | pmc = 1847610 | doi = 10.1016/j.mce.2006.12.010 }}</ref> Additional mERs besides GPER include [[ER-X]], [[ERx]], and [[Gq-mER|G<sub>q</sub>-mER]].<ref name="pmid23756388">{{cite journal | vauthors = Soltysik K, Czekaj P | title = Membrane estrogen receptors – is it an alternative way of estrogen action? | journal = Journal of Physiology and Pharmacology | volume = 64 | issue = 2 | pages = 129–42 | date = April 2013 | pmid = 23756388 }}</ref><ref name="pmid22538318">{{cite journal | vauthors = Micevych PE, Kelly MJ | title = Membrane estrogen receptor regulation of hypothalamic function | journal = Neuroendocrinology | volume = 96 | issue = 2 | pages = 103–10 | year = 2012 | pmid = 22538318 | pmc = 3496782 | doi = 10.1159/000338400 }}</ref> | |||
ERα/ERβ are in inactive state trapped in multimolecular chaperone complexes organized around the heat shock protein 90 (HSP90), containing p23 protein, and immunophilin, and located in majority in cytoplasm and partially in nucleus. In the E2 classical pathway or estrogen classical pathway, estradiol enters the [[cytoplasm]], where it interacts with ERs. Once bound E2, ERs dissociate from the molecular chaperone complexes and become competent to dimerize, migrate to nucleus, and to bind to specific DNA sequences ([[estrogen response element]], ERE), allowing for gene transcription which can take place over hours and days. | |||
Given by [[subcutaneous injection]] in mice, estradiol is about 10-fold more potent than estrone and about 100-fold more potent than estriol.<ref name="Labhart2012">{{cite book|vauthors=Labhart A|title=Clinical Endocrinology: Theory and Practice|url=https://books.google.com/books?id=DAgJCAAAQBAJ&pg=PA548|date=6 December 2012|publisher=Springer Science & Business Media|isbn=978-3-642-96158-8|pages=548–|access-date=11 November 2018|archive-date=10 January 2023|archive-url=https://web.archive.org/web/20230110014156/https://books.google.com/books?id=DAgJCAAAQBAJ&pg=PA548|url-status=live}}</ref><ref name="Blackburn2007">{{cite book|vauthors=Tucker SB|title=Maternal, Fetal, & Neonatal Physiology: A Clinical Perspective|url=https://books.google.com/books?id=2y6zOSQcn14C&pg=PA43|year=2007|publisher=Elsevier Health Sciences|isbn=978-1-4160-2944-1|pages=43–|access-date=7 June 2017|archive-date=10 January 2023|archive-url=https://web.archive.org/web/20230110014156/https://books.google.com/books?id=2y6zOSQcn14C&pg=PA43|url-status=live}}</ref><ref name="Hall2015">{{cite book| vauthors = Hall JE |title=Guyton and Hall Textbook of Medical Physiology E-Book|url=https://books.google.com/books?id=krLSCQAAQBAJ&pg=PA1043|date=31 May 2015|publisher=Elsevier Health Sciences|isbn=978-0-323-38930-3|pages=1043–}}</ref> As such, estradiol is the main estrogen in the body, although the roles of estrone and estriol as estrogens are said not to be negligible.<ref name="Hall2015" /> | |||
{{Selected biological properties of endogenous estrogens in rats}} | |||
== Biochemistry == | |||
[[File:Steroidogenesis.svg|thumb|450px|right|Human [[steroidogenesis]], showing estradiol at bottom right.<ref name="HäggströmRichfield2014">{{cite journal | vauthors = Häggström M, Richfield D | year=2014 |title=Diagram of the pathways of human steroidogenesis|journal=WikiJournal of Medicine|volume=1|issue=1|doi=10.15347/wjm/2014.005|issn=2002-4436|doi-access=free}}</ref>]] | |||
=== Biosynthesis === | |||
Estradiol, like other [[steroid hormone]]s, is derived from [[cholesterol]]. After [[side chain]] cleavage and using the Δ<sup>5</sup> or the Δ<sup>4</sup>- pathway, [[androstenedione]] is the key intermediary. A portion of the androstenedione is converted to testosterone, which in turn undergoes conversion to estradiol by aromatase. In an alternative pathway, androstenedione is [[aromaticity|aromatized]] to [[estrone]], which is subsequently converted to estradiol via [[17β-hydroxysteroid dehydrogenase]] (17β-HSD).<ref>{{cite book | vauthors = Boron WF, Boulpaep EL |title=Medical Physiology: A Cellular And Molecular Approach |publisher=Elsevier/Saunders |year=2003 |page=1300 |isbn=978-1-4160-2328-9}}</ref> | |||
During the reproductive years, most estradiol in women is produced by the [[granulosa cell]]s of the ovaries by the aromatization of androstenedione (produced in the theca folliculi cells) to estrone, followed by conversion of estrone to estradiol by 17β-HSD. Smaller amounts of estradiol are also produced by the [[adrenal cortex]], and, in men, by the testes.{{medcn|date=February 2019}} | |||
Estradiol is not produced in the [[gonad]]s only; in particular, [[adipose tissue|fat cells]] produce active precursors to estradiol, and will continue to do so even after menopause.<ref name="Mutschler">{{Cite book| vauthors = Mutschler E, Schäfer-Korting M | title = Arzneimittelwirkungen|language=de|location=Stuttgart|publisher=Wissenschaftliche Verlagsgesellschaft|year=2001|edition=8|pages=434, 444|isbn=978-3-8047-1763-3}}</ref> Estradiol is also produced in the [[brain]] and in [[artery|arterial walls]]. | |||
In men, approximately 15 to 25% of circulating estradiol is produced in the [[testicle]]s.<ref name="Melmed2016">{{cite book|vauthors=Melmed S|title=Williams Textbook of Endocrinology|url=https://books.google.com/books?id=YZ8_CwAAQBAJ&pg=PA710|date=1 January 2016|publisher=Elsevier Health Sciences|isbn=978-0-323-29738-7|pages=710–|access-date=21 March 2018|archive-date=10 January 2023|archive-url=https://web.archive.org/web/20230110014157/https://books.google.com/books?id=YZ8_CwAAQBAJ&pg=PA710|url-status=live}}</ref><ref name="MarcusFeldman2013">{{cite book| vauthors = Marcus R, Feldman D, Dempster DW, Luckey M, Cauley JA |title= Osteoporosis |url= https://books.google.com/books?id=b1FtazykqzMC&pg=PA331 |date=13 June 2013|publisher=Academic Press|isbn=978-0-12-398252-0|pages=331–}}</ref> The rest is synthesized via peripheral aromatization of testosterone into estradiol and of androstenedione into estrone (which is then transformed into estradiol via peripheral 17β-HSD).<ref name="Melmed2016" /><ref name="MarcusFeldman2013" /> This peripheral aromatization occurs predominantly in [[adipose tissue]], but also occurs in other [[tissue (biology)|tissues]] such as [[bone]], [[liver]], and the [[brain]].<ref name="Melmed2016" /> Approximately 40 to 50 μg of estradiol is produced per day in men.<ref name="Melmed2016" /> | |||
=== Distribution === | |||
In plasma, estradiol is largely bound to SHBG and [[human serum albumin|albumin]]. Only about 2.21% (± 0.04%) of estradiol is free and biologically active. The percentage remains constant throughout the [[menstrual cycle]].<ref>{{cite journal | vauthors = Wu CH, Motohashi T, Abdel-Rahman HA, Flickinger GL, Mikhail G | title = Free and protein-bound plasma estradiol-17 beta during the menstrual cycle | journal = The Journal of Clinical Endocrinology and Metabolism | volume = 43 | issue = 2 | pages = 436–45 | date = August 1976 | pmid = 950372 | doi = 10.1210/jcem-43-2-436 }}</ref> | |||
=== Metabolism === | |||
{{See also|Catechol estrogen|Estrogen conjugate|Hydroxylation of estradiol}} | |||
{{Estradiol metabolism|align=right}} | |||
Inactivation of estradiol includes conversion to less-active estrogens, such as estrone and estriol. Estriol is the major urinary [[metabolite]].{{Citation needed|date=December 2016}} Estradiol is [[conjugation (biochemistry)|conjugated]] in the [[liver]] to form [[estrogen conjugate]]s like [[estradiol sulfate]], [[estradiol glucuronide]] and, as such, excreted via the [[kidney]]s. Some of the water-soluble conjugates are excreted via the [[bile duct]], and partly reabsorbed after [[hydrolysis]] from the [[gastrointestinal tract|intestinal tract]]. This [[enterohepatic circulation]] contributes to maintaining estradiol levels. | |||
Estradiol is also metabolized via [[hydroxylation]] into [[catechol estrogen]]s. In the liver, it is non-specifically metabolized by [[CYP1A2]], [[CYP3A4]], and [[CYP2C9]] via 2-hydroxylation into [[2-hydroxyestradiol]], and by [[CYP2C9]], [[CYP2C19]], and [[CYP2C8]] via 17β-hydroxy dehydrogenation into [[estrone]],<ref name="pmid11741520">{{cite journal | vauthors = Cheng ZN, Shu Y, Liu ZQ, Wang LS, Ou-Yang DS, Zhou HH | title = Role of cytochrome P450 in estradiol metabolism in vitro | journal = Acta Pharmacologica Sinica | volume = 22 | issue = 2 | pages = 148–54 | date = February 2001 | pmid = 11741520 }}</ref> with various other [[cytochrome P450]] (CYP) [[enzyme]]s and [[metabolic reaction|metabolic transformations]] also being involved.<ref name="pmid12865317">{{cite journal | vauthors = Lee AJ, Cai MX, Thomas PE, Conney AH, Zhu BT | title = Characterization of the oxidative metabolites of 17beta-estradiol and estrone formed by 15 selectively expressed human cytochrome p450 isoforms | journal = Endocrinology | volume = 144 | issue = 8 | pages = 3382–98 | date = August 2003 | pmid = 12865317 | doi = 10.1210/en.2003-0192 | doi-access = free }}</ref> | |||
Estradiol is additionally [[conjugation (biochemistry)|conjugated]] with an [[ester]] into [[lipoidal estradiol]] forms like [[estradiol palmitate]] and [[estradiol stearate]] to a certain extent; these esters are stored in [[adipose tissue]] and may act as a very long-lasting reservoir of estradiol.<ref name="OettelSchillinger2012A">{{cite book| vauthors = Oettel M, Schillinger E |title=Estrogens and Antiestrogens I: Physiology and Mechanisms of Action of Estrogens and Antiestrogens|url=https://books.google.com/books?id=0BfrCAAAQBAJ&pg=PA235|date=6 December 2012|publisher=Springer Science & Business Media|isbn=978-3-642-58616-3|pages=235–237}}</ref><ref name="OettelSchillinger2012B">{{cite book| vauthors = Oettel M, Schillinger E |title=Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen|url=https://books.google.com/books?id=wBvyCAAAQBAJ&pg=PA268|date=6 December 2012|publisher=Springer Science & Business Media|isbn=978-3-642-60107-1|pages=268, 271}}</ref> | |||
=== Excretion === | |||
Estradiol is [[excretion|excreted]] in the form of [[glucuronide]] and [[sulfate]] [[estrogen conjugate]]s in [[urine]]. Following an [[intravenous injection]] of [[radiolabel|labeled]] estradiol in women, almost 90% is excreted in urine and [[feces]] within 4 to 5 days.<ref name="Dorfman1961">{{cite book|last1=Dorfman|first1=Ralph I.|title=Radioactive Isotopes in Physiology Diagnostics and Therapy / Künstliche Radioaktive Isotope in Physiologie Diagnostik und Therapie|chapter=Steroid Hormone Metabolism|year=1961|pages=1223–1241|doi=10.1007/978-3-642-49761-2_39|isbn=978-3-642-49477-2}}</ref><ref name="pmid13463090">{{cite journal | vauthors = Sandberg AA, Slaunwhite WR | title = Studies on phenolic steroids in human subjects. II. The metabolic fate and hepato-biliary-enteric circulation of C14-estrone and C14-estradiol in women | journal = J. Clin. Invest. | volume = 36 | issue = 8 | pages = 1266–78 | date = August 1957 | pmid = 13463090 | pmc = 1072719 | doi = 10.1172/JCI103524}}</ref> [[Enterohepatic recirculation]] causes a delay in excretion of estradiol.<ref name="Dorfman1961" /> | |||
=== Levels === | |||
[[File:Estradiol levels across the normal menstrual cycle in women.png|class=skin-invert-image|thumb|right|475px|Estradiol levels across the menstrual cycle in 36 normally cycling, ovulatory women, based on 956 specimens.<ref name="Abbott2009" /> The horizontal dashed lines are the mean integrated levels for each curve. The vertical dashed line in the center is mid-cycle.]] | |||
Levels of estradiol in premenopausal women are highly variable throughout the menstrual cycle and reference ranges widely vary from source to source.<ref name="BeckerBerkley2007">{{cite book | vauthors = Becker JB, Berkley KJ, Geary N, Hampson E, Herman JP, Young E |author-link5=James P. Herman |title=Sex Differences in the Brain: From Genes to Behavior|url=https://books.google.com/books?id=IeaLXPWsbuAC&pg=PA64|date=4 December 2007|publisher=Oxford University Press|isbn=978-0-19-804255-6|pages=64–|quote=Estradiol levels are minimal during the earliest days of the follicular phase, but increasing concentrations are released into the general circulation as the follicle matures. The highest levels are reached about 24 to 48 hours before the LH peak. In fact, the pre-ovulatory peak in estradiol represents its highest concentration during the entire menstrual cycle. Serum concentrations at this time are typically about 130–200 pg/mL, but concentrations as high as 300–400 pg/mL can be achieved in some women. Following a transient fall in association with ovulation, estradiol secretion is restored by production from the corpus luteum during the luteal phase. Plateau levels of around 100–150 pg/mL (Abraham, 1978; Thorneycroft et al., 1971) are most often seen during the period from −10 to −5 days before the onset of menses. With the regression of the corpus luteum, estradiol levels fall, gradually in some women and precipitously in others, during the last few days of the luteal phase. This ushers in the onset of menses, the sloughing of the endometrium. Serum estradiol during menses is approximately 30–50 pg/mL. (Source.)}}</ref> Estradiol levels are minimal and according to most laboratories range from 20 to 80 pg/mL during the early to mid follicular phase (or the first week of the menstrual cycle, also known as menses).<ref name="StraussBarbieri2009">{{cite book|vauthors=Strauss JR, Barbieri RL|title=Yen and Jaffe's Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management|url=https://books.google.com/books?id=NudwnhxY8kYC&pg=PA807|year=2009|publisher=Elsevier Health Sciences|isbn=978-1-4160-4907-4|pages=807–|quote=In most laboratories, serum estradiol levels range from 20 to 80 pg/mL during the early to midfollicular phase of the menstrual cycle and peak at 200 to 500 pg/mL during the preovulatory surge. During the midluteal phase, serum estradiol levels range from 60 to 200 pg/mL.|access-date=21 December 2016|archive-date=10 January 2023|archive-url=https://web.archive.org/web/20230110014157/https://books.google.com/books?id=NudwnhxY8kYC&pg=PA807|url-status=live}}</ref><ref name="ChristianSchoultz1994" /> Levels of estradiol gradually increase during this time and through the mid to late follicular phase (or the second week of the menstrual cycle) until the pre-ovulatory phase.<ref name="BeckerBerkley2007" /><ref name="StraussBarbieri2009" /> At the time of pre-ovulation (a period of about 24 to 48 hours), estradiol levels briefly surge and reach their highest concentrations of any other time during the menstrual cycle.<ref name="BeckerBerkley2007" /> Circulating levels are typically between 130 and 200 pg/mL at this time, but in some women may be as high as 300 to 400 pg/mL, and the upper limit of the reference range of some laboratories are even greater (for instance, 750 pg/mL).<ref name="BeckerBerkley2007" /><ref name="StraussBarbieri2009" /><ref name="JamesonGroot2010">{{cite book | vauthors = Jameson JL, De Groot LJ | title=Endocrinology: Adult and Pediatric | url = https://books.google.com/books?id=W4dZ-URK8ZoC&pg=PA2812|date=18 May 2010|publisher=Elsevier Health Sciences|isbn=978-1-4557-1126-0|pages=2812–|quote=Midcycle: 150-750 pg/mL}}</ref><ref name="HayWass2009">{{cite book | vauthors = Hay ID, Wass JA |title=Clinical Endocrine Oncology|url=https://books.google.com/books?id=fGio-5vtqqkC&pg=PA623|date=26 January 2009|publisher=John Wiley & Sons|isbn=978-1-4443-0023-9|pages=623–|quote=Mid-cycle: 110-330 pg/mL}}</ref><ref name="Dons1994">{{cite book| vauthors = Dons RF |title=Endocrine and Metabolic Testing Manual|url=https://books.google.com/books?id=w8jGxo_xoI4C&pg=SA8-PA8|date=12 July 1994|publisher=CRC Press|isbn=978-0-8493-7657-3|pages=8–|quote=Ovulatory: 200-400 pg/mL}}</ref> Following ovulation (or mid-cycle) and during the latter half of the menstrual cycle or the luteal phase, estradiol levels plateau and fluctuate between around 100 and 150 pg/mL during the early and mid luteal phase, and at the time of the late luteal phase, or a few days before menstruation, reach a low of around 40 pg/mL.<ref name="BeckerBerkley2007" /><ref name="ChristianSchoultz1994" /> The mean integrated levels of estradiol during a full menstrual cycle have variously been reported by different sources as 80, 120, and 150 pg/mL.<ref name="ChristianSchoultz1994">{{cite book | vauthors = Christian C, von Schoultz B| title = Hormone Replacement Therapy: Standardized or Individually Adapted Doses?|url=https://books.google.com/books?id=apU4AfUqSGwC&pg=PA60|date=15 March 1994|publisher=CRC Press|isbn=978-1-85070-545-1|pages=60–|quote=Plasma levels of estradiol range from 40 to 80 pg/mL during the 1st week of the ovarian cycle (early follicular phase) and from 80 to 300 pg/mL during the 2nd week (mid- and late follicular phase including periovulatory peak). Then during the 3rd and 4th weeks, estradiol fluctuates between 100 and 150 pg/mL (early and mid-luteal phase) to 40 pg/mL a few days before menstruation (late luteal phase). The mean integrated estradiol level during a full 28-day normal cycle is around 80 pg/mL.}}</ref><ref name="NotelovitzKeep2012">{{cite book|vauthors=Notelovitz M, van Keep PA|title=The Climacteric in Perspective: Proceedings of the Fourth International Congress on the Menopause, held at Lake Buena Vista, Florida, October 28 – November 2, 1984|url=https://books.google.com/books?id=VM0hBQAAQBAJ&pg=PA397|date=6 December 2012|publisher=Springer Science & Business Media|isbn=978-94-009-4145-8|pages=397–|quote=[...] following the menopause, circulating estradiol levels decrease from a premenopausal mean of 120 pg/mL to only 13 pg/mL.|access-date=22 October 2016|archive-date=10 January 2023|archive-url=https://web.archive.org/web/20230110014157/https://books.google.com/books?id=VM0hBQAAQBAJ&pg=PA397|url-status=live}}</ref><ref name="MüllerMacLeod2012">{{cite book|vauthors=Müller EE, MacLeod RM|title=Neuroendocrine Perspectives|url=https://books.google.com/books?id=TUXtBwAAQBAJ&pg=PA121|date=6 December 2012|publisher=Springer Science & Business Media|isbn=978-1-4612-3554-5|pages=121–|quote=[...] [premenopausal] mean [estradiol] concentration of 150 pg/mL [...]|access-date=22 October 2016|archive-date=10 January 2023|archive-url=https://web.archive.org/web/20230110014157/https://books.google.com/books?id=TUXtBwAAQBAJ&pg=PA121|url-status=live}}</ref> Although contradictory reports exist, one study found mean integrated estradiol levels of 150 pg/mL in younger women whereas mean integrated levels ranged from 50 to 120 pg/mL in older women.<ref name="MüllerMacLeod2012" /> | |||
During the reproductive years of human females, levels of estradiol are somewhat higher than that of estrone, except during the early follicular phase of the menstrual cycle; thus, estradiol may be considered the predominant estrogen during human female reproductive years in terms of absolute serum levels and estrogenic activity.{{citation needed|date=June 2014}} During pregnancy, estriol becomes the predominant circulating estrogen, and this is the only time at which estetrol occurs in the body, while during menopause, estrone predominates (both based on serum levels).{{citation needed|date=June 2014}} The estradiol produced by male humans, from testosterone, is present at serum levels roughly comparable to those of [[postmenopausal]] women (14–55 versus <35 pg/mL, respectively).{{citation needed|date=June 2014}} It has also been reported that if concentrations of estradiol in a 70-year-old man are compared to those of a 70-year-old woman, levels are approximately 2- to 4-fold higher in the man.<ref name="pmid14644018">{{cite journal | vauthors = Sayed Y, Taxel P | title = The use of estrogen therapy in men | journal = Current Opinion in Pharmacology | volume = 3 | issue = 6 | pages = 650–4 | date = December 2003 | pmid = 14644018 | doi = 10.1016/j.coph.2003.07.004}}</ref> | |||
{| class="wikitable sortable mw-collapsible" style="margin: 1em auto;" | |||
|+ class="nowrap" | Endogenous <noinclude>[[estradiol (medication)|estradiol]]</noinclude><includeonly>estradiol</includeonly> production rates and plasma <noinclude>[[estrogen (medication)|estrogen]]</noinclude><includeonly>estrogen</includeonly> levels | |||
|- | |||
! Group !! {{abbr|E2|Estradiol}} ({{abbr|prod|production rate}}) !! {{abbr|E2|Estradiol}} (levels) !! {{abbr|E1|Estrone}} (levels) !! {{abbr|Ratio|Ratio (E2:E1)}} | |||
|- | |||
| [[Puberty|Pubertal]] girls<sup>a</sup><br /> [[Tanner scale#Definitions of stages|Tanner stage I]] (childhood)<br /> [[Tanner scale#Definitions of stages|Tanner stage II]] (ages 8–12)<br /> [[Tanner scale#Definitions of stages|Tanner stage III]] (ages 10–13)<br /> [[Tanner scale#Definitions of stages|Tanner stage IV]] (ages 11–14)<br /> [[Tanner scale#Definitions of stages|Tanner stage V]] (ages 12–15)<br /> [[Follicular phase|Follicular]] (days 1–14)<br /> [[Luteal phase|Luteal]] (days 15–28) || <br />?<br />?<br />?<br />?<br /> <br />?<br />? || <br />9 (<9–20) pg/mL<br />15 (<9–30) pg/mL<br />27 (<9–60) pg/mL<br />55 (16–85) pg/mL<br /> <br />50 (30–100) pg/mL<br />130 (70–300) pg/mL || <br />13 (<9–23) pg/mL<br />18 (10–37) pg/mL<br />26 (17–58) pg/mL<br />36 (23–69) pg/mL<br /> <br />44 (30–89) pg/mL<br />75 (39–160) pg/mL || <br />?<br />?<br />?<br />?<br /> <br />?<br />? | |||
|- | |||
| [[Prepuberty|Prepubertal]] boys || ? || 2–8 pg/mL || ? || ? | |||
|- | |||
| [[Premenopause|Premenopausal]] women<br /> [[Follicular phase|Early follicular phase]] (days 1–4)<br /> [[Follicular phase|Mid follicular phase]] (days 5–9)<br /> [[Follicular phase|Late follicular phase]] (days 10–14)<br /> [[Luteal phase]] (days 15–28)<br /> [[Oral contraceptive]] ([[anovulation|anovulatory]]) || <br />30–100 μg/day<br />100–160 μg/day<br />320–640 μg/day<br />300 μg/day<br />? || <br />40–60 pg/mL<br />60–100 pg/mL<br />200–400 pg/mL<br />190 pg/mL<br />12–50 pg/mL || <br />40–60 pg/mL<br />?<br />170–200 pg/mL<br />100–150 pg/mL<br />? || <br />0.5–1<br />?<br />1–2<br />1.5<br />? | |||
|- | |||
| [[Menopause|Postmenopausal]] women || 18 μg/day || 5–20 pg/mL || 30–70 pg/mL || 0.3–0.8 | |||
|- | |||
| [[Pregnancy|Pregnant]] women<br /> [[First trimester]] (weeks 1–12)<br /> [[Second trimester]] (weeks 13–26)<br /> [[Third trimester]] (weeks 27–40) || <br />?<br />?<br />? || <br />1,000–5,000 pg/mL<br />5,000–15,000 pg/mL<br />10,000–40,000 pg/mL || <br />?<br />?<br />? || <br />?<br />?<br />? | |||
|- | |||
| Men<sup>a</sup> || 20–60 μg/day || 27 (20–55) pg/mL || 20–90 pg/mL || 0.4–0.6 | |||
|- class="sortbottom" | |||
| colspan="5" style="width: 1px; background-color:#eaecf0; text-align: center;" | '''Footnotes:''' <sup>a</sup> = Format is "mean value (range)" or just "range". '''Sources:''' <noinclude><ref name="pmid6717863">{{cite journal | vauthors = Nichols KC, Schenkel L, Benson H | title = 17 beta-estradiol for postmenopausal estrogen replacement therapy | journal = Obstet Gynecol Surv | volume = 39 | issue = 4 | pages = 230–45 | year = 1984 | pmid = 6717863 | doi = 10.1097/00006254-198404000-00022 }}</ref><ref name="CherneckyBerger2012">{{cite book|author1=Cynthia C. Chernecky|author2=Barbara J. Berger|title=Laboratory Tests and Diagnostic Procedures – E-Book|url=https://books.google.com/books?id=dWHYcOJK-cgC&pg=PA488|date=31 October 2012|publisher=Elsevier Health Sciences|isbn=978-1-4557-4502-9|pages=488–|access-date=22 August 2023|archive-date=22 August 2023|archive-url=https://web.archive.org/web/20230822235743/https://books.google.com/books?id=dWHYcOJK-cgC&pg=PA488|url-status=live}}</ref><ref name="pmid2992279">{{cite journal | vauthors = Powers MS, Schenkel L, Darley PE, Good WR, Balestra JC, Place VA | title = Pharmacokinetics and pharmacodynamics of transdermal dosage forms of 17 beta-estradiol: comparison with conventional oral estrogens used for hormone replacement | journal = Am. J. Obstet. Gynecol. | volume = 152 | issue = 8 | pages = 1099–106 | date = August 1985 | pmid = 2992279 | doi = 10.1016/0002-9378(85)90569-1 }}</ref><ref name="Becker2001">{{cite book|author=Kenneth L. Becker|title=Principles and Practice of Endocrinology and Metabolism|url=https://books.google.com/books?id=FVfzRvaucq8C&pg=PA1059|year=2001|publisher=Lippincott Williams & Wilkins|isbn=978-0-7817-1750-2|pages=889, 1059–1060, 2153}}</ref><ref name="BajajBerman2011" /><ref name="Abbott2009" /><ref name="Kuhl2003" /></noinclude><includeonly>See template.</includeonly> | |||
|} | |||
==== Measurement ==== | |||
In women, serum estradiol is measured in a [[clinical laboratory]] and reflects primarily the activity of the ovaries. The Estradiol blood test measures the amount of estradiol in the blood.<ref name=":0" /> It is used to check the function of the ovaries, placenta, adrenal glands.<ref name=":0" /> This can detect baseline estrogen in women with [[amenorrhea]] or menstrual dysfunction, and to detect the state of hypoestrogenicity and menopause. Furthermore, estrogen monitoring during fertility therapy assesses follicular growth and is useful in monitoring the treatment. Estrogen-producing tumors will demonstrate persistent high levels of estradiol and other estrogens. In [[precocious puberty]], estradiol levels are inappropriately increased. | |||
==== Ranges ==== | |||
Individual laboratory results should always be interpreted using the ranges provided by the laboratory that performed the test. | |||
{| class="wikitable" align="left" | |||
|+ [[Reference ranges for blood tests|Reference ranges for serum]] estradiol | |||
|- | |||
! Patient type | |||
! Lower limit | |||
! Upper limit | |||
! Unit | |||
|- | |||
| rowspan=2| Adult male || 50<ref name="gpnotebook-estradiol">[http://www.gpnotebook.co.uk/simplepage.cfm?ID=570818627&linkID=24801&cook=yes GPNotebook — reference range (oestradiol)] {{Webarchive|url=https://web.archive.org/web/20120609174939/http://www.gpnotebook.co.uk/simplepage.cfm?ID=570818627&linkID=24801&cook=yes |date=9 June 2012 }} Retrieved on 27 September 2009</ref> || 200<ref name="gpnotebook-estradiol" /> || pmol/L | |||
|- | |||
| 14 || 55 || pg/mL | |||
|- | |||
| rowspan=4| Adult female ([[Follicular phase|follicular<br /> phase]], day 5) || 70<ref name="gpnotebook-estradiol" /><br /><span style="font-size:87%;">95% [[prediction interval|PI]] (standard)</span> || 500<ref name="gpnotebook-estradiol" /><br /><span style="font-size:87%;">95% PI</span> || rowspan=2 | pmol/L | |||
|- | |||
| 110<ref name=Stricker>Values taken from day 1 after LH surge in: {{cite journal | vauthors = Stricker R, Eberhart R, Chevailler MC, Quinn FA, Bischof P, Stricker R | title = Establishment of detailed reference values for luteinizing hormone, follicle stimulating hormone, estradiol, and progesterone during different phases of the menstrual cycle on the Abbott ARCHITECT analyzer | journal = Clinical Chemistry and Laboratory Medicine | volume = 44 | issue = 7 | pages = 883–7 | year = 2006 | pmid = 16776638 | doi = 10.1515/CCLM.2006.160 | s2cid = 524952 }} [https://web.archive.org/web/20110706224350/https://www.dianalabs.ch/documents/ajouts/Hormones.pdf as PDF]</ref><br /><span style="font-size:87%;">90% [[prediction interval|PI]] (used <br />in [[:File:Estradiol during menstrual cycle.png|diagram]])</span> || 220<ref name=Stricker /><br /><span style="font-size:87%;">90% PI</span> | |||
|- | |||
| 19 <span style="font-size:87%;">(95% PI)</span> || 140 <span style="font-size:87%;">(95% PI)</span> || rowspan=2 | pg/mL | |||
|- | |||
| 30 <span style="font-size:87%;">(90% PI)</span> || 60 <span style="font-size:87%;">(90% PI)</span> | |||
|- | |||
| rowspan=2| Adult female ([[preovulatory]]<br /> peak) || 400<ref name="gpnotebook-estradiol" /> || 1500<ref name="gpnotebook-estradiol" /> || pmol/L | |||
|- | |||
| 110 || 410 || pg/mL | |||
|- | |||
| rowspan=2| Adult female <br />([[luteal phase]]) || 70<ref name="gpnotebook-estradiol" /> || 600<ref name="gpnotebook-estradiol" /> || pmol/L | |||
|- | |||
| 19 || 160 || pg/mL | |||
|- | |||
| rowspan=2| Adult female – free <br />(not protein bound) || 0.5<ref name="free-estradiol">Total amount multiplied by 0.022 according to 2.2% presented in: {{cite journal | vauthors = Wu CH, Motohashi T, Abdel-Rahman HA, Flickinger GL, Mikhail G | title = Free and protein-bound plasma estradiol-17 beta during the menstrual cycle | journal = The Journal of Clinical Endocrinology and Metabolism | volume = 43 | issue = 2 | pages = 436–45 | date = August 1976 | pmid = 950372 | doi = 10.1210/jcem-43-2-436 }}{{Original research inline|date=June 2014}}</ref>{{Original research inline|date=June 2014}} || 9<ref name="free-estradiol" />{{Original research inline|date=June 2014}} || pg/mL | |||
|- | |||
| 1.7<ref name="free-estradiol" />{{Original research inline|date=June 2014}} || 33<ref name="free-estradiol" />{{Original research inline|date=June 2014}} || pmol/L | |||
|- | |||
| rowspan=2| Post-menopausal female || N/A<ref name="gpnotebook-estradiol" /> || < 130<ref name="gpnotebook-estradiol" /> || pmol/L | |||
|- | |||
| N/A || < 35 || pg/mL | |||
|- | |||
|} | |||
{{clear}} | |||
{{Hidden begin|toggle=left|title=Reference ranges for the blood content of estradiol during the menstrual cycle}} | |||
[[File:Estradiol during menstrual cycle.png|thumb|left|upright=4.75|[[Reference ranges for blood tests|Reference ranges for the blood content]] of estradiol during the [[menstrual cycle]] | |||
<br />- The ranges denoted '''By biological stage''' may be used in closely monitored menstrual cycles in regard to other markers of its biological progression, with the time scale being compressed or stretched to how much faster or slower, respectively, the cycle progresses compared to an average cycle. | |||
<br />- The ranges denoted '''Inter-cycle variability''' are more appropriate to use in unmonitored cycles with only the beginning of menstruation known, but where the woman accurately knows her average cycle lengths and time of ovulation, and that they are somewhat averagely regular, with the time scale being compressed or stretched to how much a woman's average cycle length is shorter or longer, respectively, than the average of the population. | |||
<br />- The ranges denoted '''Inter-woman variability''' are more appropriate to use when the average cycle lengths and time of ovulation are unknown, but only the beginning of menstruation is given.<ref name="Häggström2014">{{cite journal|year=2014|title=Reference ranges for estradiol, progesterone, luteinizing hormone and follicle-stimulating hormone during the menstrual cycle|journal=WikiJournal of Medicine|volume=1|issue=1|doi=10.15347/wjm/2014.001|issn=2002-4436| vauthors = Häggström M |doi-access=free}}</ref>]] | |||
{{Hidden end}} | |||
In the normal menstrual cycle, estradiol levels measure typically <50 pg/mL at menstruation, rise with follicular development (peak: 200 pg/mL), drop briefly at ovulation, and rise again during the luteal phase for a second peak. At the end of the luteal phase, estradiol levels drop to their menstrual levels unless there is a pregnancy. | |||
During pregnancy, estrogen levels, including estradiol, rise steadily toward term. The source of these estrogens is the [[placenta]], which aromatizes [[prohormone]]s produced in the fetal adrenal gland. | |||
{{Production rates, secretion rates, clearance rates, and blood levels of major sex hormones}} | |||
== Medical use == | |||
{{Main|Estradiol (medication)|Pharmacodynamics of estradiol|Pharmacokinetics of estradiol}} | |||
Estradiol is used as a [[medication]], primarily in [[hormone replacement therapy|hormone therapy]] for [[menopause|menopausal]] [[symptom]]s as well as [[feminizing hormone therapy]] for trans individuals.<ref name="pmid16112947" /> | |||
== Chemistry == | |||
{{See also|List of estrogens}} | |||
{{Chemical structures of major endogenous estrogens|align=right|caption=Note the [[hydroxyl group|hydroxyl]] (–OH) [[functional group|groups]]: estrone (E1) has one, estradiol (E2) has two, estriol (E3) has three, and estetrol (E4) has four.}} | |||
Estradiol is an [[estrane]] [[steroid]].<ref name="pmid16112947">{{cite journal | vauthors = Kuhl H | s2cid = 24616324 | title = Pharmacology of estrogens and progestogens: influence of different routes of administration | journal = Climacteric | volume = 8 | issue = 1 Suppl 1 | pages = 3–63 | date = August 2005 | pmid = 16112947 | doi = 10.1080/13697130500148875 }}</ref> It is also known as 17β-estradiol (to distinguish it from [[17α-estradiol]]) or as estra-1,3,5(10)-triene-3,17β-diol. It has two [[hydroxyl group]]s, one at the C3 position and the other at the 17β position, as well as three [[double bond]]s in the A [[ring (chemistry)|ring]]. Due to its two hydroxyl groups, estradiol is often abbreviated as E2. The structurally related estrogens, estrone (E1), estriol (E3), and [[estetrol]] (E4) have one, three, and four hydroxyl groups, respectively. | |||
== Neuropsychopharmacology == | |||
Product insert information, accompanying commercial perscription estradiol, indicates it causes depression. | |||
In a randomized, double-blind, placebo-controlled study, estradiol was shown to have gender-specific effects on fairness sensitivity. Overall, when the division of a given amount of money was framed as either fair or unfair in a modified version of the [[ultimatum game]], estradiol increased the acceptance rate of fair-framed proposals among men and decreased it among women. However, among the placebo-group "the mere belief of receiving estradiol treatment significantly increased the acceptance of unfair-framed offers in both sexes", indicating that so-called "environmental" factors played a role in organising the responses towards these presentations of the [[ultimatum game]].<ref>{{cite journal | vauthors = Coenjaerts M, Pape F, Santoso V, Grau F, Stoffel-Wagner B, Philipsen A, Schultz J, Hurlemann R, Scheele D |title=Sex differences in economic decision-making: Exogenous estradiol has opposing effects on fairness framing in women and men. |journal=Eur. Neuropsychopharmacol. |volume=50 |issue=2 |pages=46–54 |date= September 2021|pmid=33957337|doi= 10.1016/j.euroneuro.2021.04.006 | issn=0924-977X|s2cid=233982738 |hdl=20.500.11811/11066 |hdl-access=free }}</ref> | |||
== History == | |||
{{See also|Estrone#History}} | |||
The discovery of estrogen is usually credited to the [[United States|American]] [[scientist]]s [[Edgar Allen]] and [[Edward Adelbert Doisy|Edward A. Doisy]].<ref name="LoriauxLoriaux2016">{{cite book | vauthors = Loriaux DL, Loriaux L |title=A Biographical History of Endocrinology|url=https://books.google.com/books?id=pkWhCwAAQBAJ&pg=PA345|date=14 March 2016|publisher=John Wiley & Sons|isbn=978-1-119-20246-2|pages=345–}}</ref><ref name="LauritzenStudd2005">{{cite book | vauthors = Lauritzen C, Studd JW |title=Current Management of the Menopause|url=https://books.google.com/books?id=WD7S7677xUUC&pg=PA44|date=22 June 2005|publisher=CRC Press|isbn=978-0-203-48612-2|pages=44–}}</ref> In 1923, they observed that injection of fluid from [[porcine]] [[ovarian follicle]]s produced [[puberty|pubertal]]- and [[estrus]]-type changes (including [[vagina]]l, [[uterus|uterine]], and [[mammary gland]] changes and [[sexual receptivity]]) in [[sexual maturity|sexually immature]], [[ovariectomy|ovariectomized]] mice and rats.<ref name="LoriauxLoriaux2016" /><ref name="LauritzenStudd2005" /><ref name="AllenDoisy1923">{{cite journal | vauthors = Allen E, Doisy EA | title = An Ovarian Hormone | journal = Journal of the American Medical Association |volume=81 |issue=10 |year=1923 |pages=819 |issn=0002-9955 |doi=10.1001/jama.1923.02650100027012 }}</ref> These findings demonstrated the existence of a [[hormone]] which is produced by the [[ovaries]] and is involved in [[sexual maturation]] and [[sexual reproduction|reproduction]].<ref name="LoriauxLoriaux2016" /><ref name="LauritzenStudd2005" /><ref name="AllenDoisy1923" /> At the time of its discovery, Allen and Doisy did not name the hormone, and simply referred to it as an "ovarian hormone" or "follicular hormone";<ref name="LauritzenStudd2005" /> others referred to it variously as ''feminin'', ''folliculin'', ''menformon'', ''thelykinin'', and ''emmenin''.<ref name="GruhnKazer2013">{{cite book | vauthors = Gruhn JG, Kazer RR |title=Hormonal Regulation of the Menstrual Cycle: The Evolution of Concepts|url=https://books.google.com/books?id=lFn0BwAAQBAJ&pg=PA69|date=11 November 2013|publisher=Springer Science & Business Media|isbn=978-1-4899-3496-3|pages=69–73}}</ref><ref name="Newerla1944">{{cite journal| vauthors = Newerla GJ |title=The History of the Discovery and Isolation of the Female Sex Hormones|journal=New England Journal of Medicine|volume=230|issue=20|year=1944|pages=595–604|issn=0028-4793|doi=10.1056/NEJM194405182302001}}</ref> In 1926, Parkes and Bellerby coined the term ''estrin'' to describe the hormone on the basis of it inducing [[estrus]] in animals.<ref name="FritzSperoff2012">{{cite book | vauthors = Fritz MA, Speroff L |title=Clinical Gynecologic Endocrinology and Infertility|url=https://books.google.com/books?id=KZLubBxJEwEC&pg=PA750|date=28 March 2012|publisher=Lippincott Williams & Wilkins|isbn=978-1-4511-4847-3|pages=750–}}</ref><ref name="GruhnKazer2013" /> [[Estrone]] was isolated and purified independently by Allen and Doisy and [[Germany|German]] scientist [[Adolf Butenandt]] in 1929, and [[estriol]] was isolated and purified by Marrian in 1930; they were the first estrogens to be identified.<ref name="LauritzenStudd2005" /><ref name="Parl2000">{{cite book|vauthors=Parl FF|title=Estrogens, Estrogen Receptor and Breast Cancer|url=https://books.google.com/books?id=v7ai5Mz9TZQC&pg=PA4|year=2000|publisher=IOS Press|isbn=978-0-9673355-4-4|pages=4–|access-date=27 November 2016|archive-date=10 January 2023|archive-url=https://web.archive.org/web/20230110014157/https://books.google.com/books?id=v7ai5Mz9TZQC&pg=PA4|url-status=live}}</ref><ref name="SartorelliJohns2013">{{cite book | vauthors = Sartorelli AC, Johns DG |title=Antineoplastic and Immunosuppressive Agents|url=https://books.google.com/books?id=aU_oCAAAQBAJ&pg=PA104|date=27 November 2013|publisher=Springer Science & Business Media|isbn=978-3-642-65806-8|pages=104–}}</ref> | |||
Estradiol, the most potent of the three major estrogens, was the last of the three to be identified.<ref name="LauritzenStudd2005" /><ref name="FritzSperoff2012" /> It was discovered by Schwenk and Hildebrant in 1933, who [[chemical synthesis|synthesized]] it via [[redox|reduction]] of estrone.<ref name="LauritzenStudd2005" /> Estradiol was subsequently isolated and purified from sow ovaries by Doisy in 1935, with its [[chemical structure]] determined simultaneously,<ref name="ShoupeHaseltine2012">{{cite book | vauthors = Shoupe D, Haseltine FP |title=Contraception|url=https://books.google.com/books?id=cpDhBwAAQBAJ&pg=PA2|date=6 December 2012|publisher=Springer Science & Business Media|isbn=978-1-4612-2730-4|pages=2–}}</ref> and was referred to variously as ''dihydrotheelin'', ''dihydrofolliculin'', ''dihydrofollicular hormone'', and ''dihydroxyestrin''.<ref name="LauritzenStudd2005" /><ref name="MacCorquodaleThayer1935">{{cite journal | vauthors = MacCorquodale DW, Thayer SA, Doisy EA |title=The Crystalline Ovarian Follicular Hormone|journal=Experimental Biology and Medicine|volume=32|issue=7|year=1935|pages=1182|issn=1535-3702|doi=10.3181/00379727-32-8020P|s2cid=83557813}}</ref><ref name="EPA1981">{{cite book|title=Chemicals Identified in Human Biological Media: A Data Base|url=https://books.google.com/books?id=Gr-0CeNE0Z8C&pg=PA114|year=1981|publisher=Design and Development Branch, Survey and Analysis Division, Office of Program Integration and Information, Office of Pesticides and Toxic Substances, Environmental Protection Agency|pages=114–}}</ref> In 1935, the name ''estradiol'' and the term ''estrogen'' were formally established by the Sex Hormone Committee of the Health Organization of the [[League of Nations]]; this followed the names estrone (which was initially called theelin, progynon, folliculin, and ketohydroxyestrin) and estriol (initially called theelol and trihydroxyestrin) having been established in 1932 at the first meeting of the International Conference on the Standardization of Sex Hormones in [[London]].<ref name="FritzSperoff2012" /><ref name="Fausto-Sterling2000">{{cite book| vauthors = Fausto-Sterling A |title=Sexing the Body: Gender Politics and the Construction of Sexuality|url=https://archive.org/details/isbn_9780465077137|url-access=registration|year=2000|publisher=Basic Books|isbn=978-0-465-07714-4|pages=[https://archive.org/details/isbn_9780465077137/page/189 189]–}}</ref> Following its discovery, a [[partial synthesis]] of estradiol from [[cholesterol]] was developed by Inhoffen and Hohlweg in 1940, and a [[total synthesis]] was developed by Anner and Miescher in 1948.<ref name="LauritzenStudd2005" /> | |||
== Society and culture == | |||
=== Etymology === | |||
The name ''estradiol'' derives from ''{{not a typo|estra-}}'', [[Greek language|Gk.]] ''{{lang|grc|οἶστρος}}'' ({{transl|grc|oistros}}, literally meaning "verve or inspiration"),<ref>{{cite web|url=https://www.perseus.tufts.edu/hopper/morph?l=oistros&la=greek|title=Greek Word Study Tool: oistros|publisher=[[Perseus Project|Perseus Digital Library]]|access-date=28 December 2011|archive-date=17 March 2012|archive-url=https://web.archive.org/web/20120317152123/http://www.perseus.tufts.edu/hopper/morph?l=oistros&la=greek|url-status=live}}</ref> which refers to the [[estrane]] [[steroid]] [[ring (chemistry)|ring]] system, and ''{{not a typo|-diol}}'', a chemical term and suffix indicating that the compound is a type of [[Alcohol (chemistry)|alcohol]] bearing two [[hydroxyl]] [[functional group|groups]]. | |||
== References == | |||
{{reflist|refs= | |||
<ref name=":0">{{cite web |url=https://medlineplus.gov/ency/article/003711.htm |title=Estradiol blood test: MedlinePlus Medical Encyclopedia |website=medlineplus.gov |language=en |access-date=2019-05-06 |archive-date=18 March 2021 |archive-url=https://web.archive.org/web/20210318225130/https://medlineplus.gov/ency/article/003711.htm |url-status=live }}</ref> | |||
<ref name=Abbott2009>{{cite web |title=Estradiol |url=https://www.ilexmedical.com/files/PDF/Estradiol_ARC.pdf |website=ilexmedical.com |access-date=4 July 2024 |archive-date=4 February 2024 |archive-url=https://web.archive.org/web/20240204065541/https://www.ilexmedical.com/files/PDF/Estradiol_ARC.pdf |url-status=live }}</ref> | |||
<ref name="BajajBerman2011">{{cite book |author1=Lalit Bajaj |author2=Stephen Berman |title=Berman's Pediatric Decision Making |url=https://books.google.com/books?id=NPhnHrDQ1_kC&pg=PA160 |date=1 January 2011 |publisher=Elsevier Health Sciences |isbn=978-0-323-05405-8 |pages=160– |access-date=22 August 2023 |archive-date=11 January 2023 |archive-url=https://web.archive.org/web/20230111143033/https://books.google.com/books?id=NPhnHrDQ1_kC&pg=PA160 |url-status=live }}</ref> | |||
<ref name="CraigStitzel2004">{{cite book |vauthors=Craig CR, Stitzel RE |title=Modern Pharmacology with Clinical Applications |url=https://books.google.com/books?id=KqA29hQ-m3AC&pg=PA706 |year=2004 |publisher=Lippincott Williams & Wilkins |isbn=978-0-7817-3762-3 |pages=706–}}</ref> | |||
<ref name="FalconeHurd2007">{{cite book |vauthors=Falcone T, Hurd WW |title=Clinical Reproductive Medicine and Surgery |url=https://books.google.com/books?id=fOPtaEIKvcIC&pg=PA22 |year=2007 |publisher=Elsevier Health Sciences |isbn=978-0-323-03309-1 |pages=22– |access-date=22 October 2016 |archive-date=10 January 2023 |archive-url=https://web.archive.org/web/20230110014156/https://books.google.com/books?id=fOPtaEIKvcIC&pg=PA22 |url-status=live }}</ref> | |||
<ref name="FordRoach2013">{{cite book |vauthors=Ford SR, Roach SS |title=Roach's Introductory Clinical Pharmacology |url=https://books.google.com/books?id=LwOaAgAAQBAJ&pg=PA525 |date=7 October 2013 |publisher=Lippincott Williams & Wilkins |isbn=978-1-4698-3214-2 |pages=525–}}</ref> | |||
<ref name="HochadelMosby2015">{{cite book |vauthors=Hochadel M |title=Mosby's Drug Reference for Health Professions |url=https://books.google.com/books?id=IuF1BwAAQBAJ&pg=PA602 |date=1 April 2015 |publisher=Elsevier Health Sciences |isbn=978-0-323-31103-8 |pages=602–}}</ref> | |||
<ref name="Kuhl2003">{{cite journal |vauthors=Kuhl H |title=Östrogene für den Mann? |trans-title=Estrogens for the man? |journal=Blickpunkt der Mann |year=2003 |volume=1 |issue=3 |pages=6–12 |issn=1727-0669 |url=https://www.kup.at/journals/summary/3583.html |access-date=22 August 2023 |archive-date=22 August 2023 |archive-url=https://web.archive.org/web/20230822235745/https://www.kup.at/journals/summary/3583.html |url-status=live }}</ref> | |||
<ref name="McMillanFeigin2006">{{cite book |vauthors=McMillan JA, Feigin RD, DeAngelis C, Jones MD |title=Oski's Pediatrics: Principles & Practice |url=https://books.google.com/books?id=VbjFQiz8aR0C&pg=PA550 |year=2006 |publisher=Lippincott Williams & Wilkins |isbn=978-0-7817-3894-1 |pages=550–}}</ref> | |||
<ref name="Mechoulam_2005">{{cite journal |vauthors=Mechoulam R, Brueggemeier RW, Denlinger DL |title=Estrogens in insects |journal=Cellular and Molecular Life Sciences |date=September 1984 |volume=40 |issue=9 |pages=942–944 |doi=10.1007/BF01946450 |s2cid=31950471}}</ref> | |||
<ref name="NauntonAl Hadithy2006">{{cite journal |vauthors=Naunton M, Al Hadithy AF, Brouwers JR, Archer DF |title=Estradiol gel: review of the pharmacology, pharmacokinetics, efficacy, and safety in menopausal women |journal=Menopause |volume=13 |issue=3 |pages=517–27 |year=2006 |pmid=16735950 |doi=10.1097/01.gme.0000191881.52175.8c |s2cid=42748448}}</ref> | |||
<ref name="Ozon_1972">{{cite book |editor=Idler DR |title=Steroids In Nonmammalian Vertebrates |date=1972 |publisher=Elsevier Science |location=Oxford |isbn=978-0-323-14098-0 |vauthors=Ozon R |chapter=Estrogens in Fishes, Amphibians, Reptiles, and Birds |pages=390–414 |chapter-url=https://books.google.com/books?id=Ei46GE-lj2wC&q=estradiol%20vertebrates&pg=PA393 |access-date=17 October 2020 |archive-date=10 January 2023 |archive-url=https://web.archive.org/web/20230110014156/https://books.google.com/books?id=Ei46GE-lj2wC&q=estradiol%20vertebrates&pg=PA393 |url-status=live }}</ref> | |||
<ref name="pmid7083198">{{cite journal |vauthors=Ryan KJ |title=Biochemistry of aromatase: significance to female reproductive physiology |journal=Cancer Research |volume=42 |issue=8 Suppl |pages=3342s–3344s |date=August 1982 |pmid=7083198}}</ref> | |||
<ref name="Preedy2011">{{cite book |vauthors=Preedy VR |title=Handbook of Growth and Growth Monitoring in Health and Disease |url=https://books.google.com/books?id=9FQXunlUvz0C&pg=PA2661 |date=2 December 2011 |publisher=Springer Science & Business Media |isbn=978-1-4419-1794-2 |pages=2661– |access-date=9 June 2017 |archive-date=10 January 2023 |archive-url=https://web.archive.org/web/20230110014156/https://books.google.com/books?id=9FQXunlUvz0C&pg=PA2661 |url-status=live }}</ref> | |||
<ref name="PriceBlauer1997">{{cite journal |vauthors=Price TM, Blauer KL, Hansen M, Stanczyk F, Lobo R, Bates GW |title=Single-dose pharmacokinetics of sublingual versus oral administration of micronized 17 beta-estradiol |journal=Obstetrics and Gynecology |volume=89 |issue=3 |pages=340–5 |date=March 1997 |pmid=9052581 |doi=10.1016/S0029-7844(96)00513-3 |s2cid=71641652}}</ref> | |||
<ref name="StanczykArcher2013">{{cite journal |vauthors=Stanczyk FZ, Archer DF, Bhavnani BR |title=Ethinyl estradiol and 17β-estradiol in combined oral contraceptives: pharmacokinetics, pharmacodynamics and risk assessment |journal=Contraception |volume=87 |issue=6 |pages=706–27 |date=June 2013 |pmid=23375353 |doi=10.1016/j.contraception.2012.12.011}}</ref> | |||
}} | |||
{{Estradiol salts}} | |||
{{Estradiol}} | |||
{{Hormones}} | |||
{{Endogenous steroids}} | |||
{{Estrogen receptor modulators}} | |||
{{Portal bar | Medicine}} | |||
{{Authority control}} | |||
[[Category:Estradiol| ]] | |||
[[Category:Secondary alcohols]] | |||
[[Category:Animal reproductive system]] | |||
[[Category:Antigonadotropins]] | |||
[[Category:Antioxidants]] | |||
[[Category:Estranes]] | |||
[[Category:Estrogens]] | |||
[[Category:GPER agonists]] | |||
[[Category:Hepatotoxins]] | |||
[[Category:Hormones of the hypothalamus-pituitary-gonad axis]] | |||
[[Category:Hormones of the hypothalamic-pituitary-prolactin axis]] | |||
[[Category:Hormones of the pregnant female]] | |||
[[Category:Human female endocrine system]] | |||
[[Category:Hydroxyarenes]] | |||
[[Category:Prolactin releasers]] | |||
[[Category:Sex hormones]] | |||
2025年3月18日 (火) 19:29時点における版
テンプレート:Chembox MagSusテンプレート:Chembox ProteinBoundテンプレート:Chembox Excretionテンプレート:Chembox Licence
| Estradiol | |
|---|---|
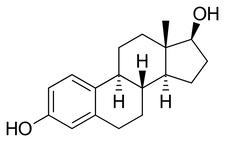
| |
| Estradiol 3D ball.png | |
Estra-1,3,5(10)-triene-3,17β-diol | |
(1S,3aS,3bR,9bS,11aS)-11a-Methyl-2,3,3a,3b,4,5,9b,10,11,11a-decahydro-1H-cyclopenta[a]phenanthrene-1,7-diol | |
別称 Oestradiol; E2; 17β-Estradiol; 17β-Oestradiol | |
| Identifiers | |
| 50-28-2 | |
| ChEBI | |
| ChEMBL | ChEMBL135 |
| ChemSpider | 5554 |
| DrugBank | {{{value}}} |
| EC-number | [1] |
| |
| Jmol-3D images | Image |
| KEGG | D00105 |
| PubChem | 5757 |
| |
| UNII | 4TI98Z838E |
| Properties | |
| C18H24O2 | |
| Molar mass | 272.38 g/mol g·mol−1 |
| Pharmacology | |
| ATC code | G03 |
| Bioavailability | Oral: <5%[1] |
| Routes of administration |
Oral, sublingual, intranasal, topical/transdermal, vaginal, intramuscular or subcutaneous (as an ester), subdermal implant |
| Metabolism | Liver (via hydroxylation, sulfation, glucuronidation) |
| Elimination half-life |
Oral: 13–20 hours[1] Sublingual: 8–18 hours[3] Topical (gel): 36.5 hours[4] |
| 特記なき場合、データは常温(25 °C)・常圧(100 kPa)におけるものである。 | |
Estradiol (E2), also called oestrogen, oestradiol, is an estrogen steroid hormone and the major female sex hormone. It is involved in the regulation of female reproductive cycles such as estrous and menstrual cycles. Estradiol is responsible for the development of female secondary sexual characteristics such as the breasts, widening of the hips and a female pattern of fat distribution. It is also important in the development and maintenance of female reproductive tissues such as the mammary glands, uterus and vagina during puberty, adulthood and pregnancy.[5] It also has important effects in many other tissues including bone, fat, skin, liver, and the brain.
Though estradiol levels in males are much lower than in females, estradiol has important roles in males as well. Apart from humans and other mammals, estradiol is also found in most vertebrates and crustaceans, insects, fish, and other animal species.[6][7]
Estradiol is produced within the follicles of the ovaries and in other tissues including the testicles, the adrenal glands, fat, liver, the breasts, and the brain. Estradiol is produced in the body from cholesterol through a series of reactions and intermediates.[8] The major pathway involves the formation of androstenedione, which is then converted by aromatase into estrone and is subsequently converted into estradiol. Alternatively, androstenedione can be converted into testosterone, which can then be converted into estradiol. Upon menopause in females, production of estrogens by the ovaries stops and estradiol levels decrease to very low levels.
In addition to its role as a natural hormone, estradiol is used as a medication, for instance in menopausal hormone therapy, and feminizing hormone therapy for transgender women and other genderqueer individuals; for information on estradiol as a medication, see the estradiol (medication) article.
Biological function
Sexual development
The development of secondary sex characteristics in women is driven by estrogens, to be specific, estradiol.[9][10] These changes are initiated at the time of puberty, most are enhanced during the reproductive years, and become less pronounced with declining estradiol support after menopause. Thus, estradiol produces breast development, and is responsible for changes in the body shape, affecting bones, joints, and fat deposition.[9][10] In females, estradiol induces breast development, widening of the hips, a feminine fat distribution (with fat deposited particularly in the breasts, hips, thighs, and buttocks), and maturation of the vagina and vulva, whereas it mediates the pubertal growth spurt (indirectly via increased growth hormone secretion)[11] and epiphyseal closure (thereby limiting final height) in both sexes.[9][10]
Reproduction
Female reproductive system
In the female, estradiol acts as a growth hormone for tissue of the reproductive organs, supporting the lining of the vagina, the cervical glands, the endometrium, and the lining of the fallopian tubes. It enhances growth of the myometrium. Estradiol appears necessary to maintain oocytes in the ovary. During the menstrual cycle, estradiol produced by the growing follicles triggers, via a positive feedback system, the hypothalamic-pituitary events that lead to the luteinizing hormone surge, inducing ovulation. In the luteal phase, estradiol, in conjunction with progesterone, prepares the endometrium for implantation. During pregnancy, estradiol increases due to placental production. The effect of estradiol, together with estrone and estriol, in pregnancy is less clear. They may promote uterine blood flow, myometrial growth, stimulate breast growth and at term, promote cervical softening and expression of myometrial oxytocin receptors.[citation needed] In baboons, blocking of estrogen production leads to pregnancy loss, suggesting estradiol has a role in the maintenance of pregnancy. Research is investigating the role of estrogens in the process of initiation of labor. Actions of estradiol are required before the exposure of progesterone in the luteal phase.[citation needed]
Male reproductive system
The effect of estradiol (and estrogens in general) upon male reproduction is complex. Estradiol is produced by action of aromatase mainly in the Leydig cells of the mammalian testis, but also by some germ cells and the Sertoli cells of immature mammals.[12] It functions (in vitro) to prevent apoptosis of male sperm cells.[13] While some studies in the early 1990s claimed a connection between globally declining sperm counts and estrogen exposure in the environment,[14] later studies found no such connection, nor evidence of a general decline in sperm counts.[15][16] Suppression of estradiol production in a subpopulation of subfertile men may improve the semen analysis.[17]
Males with certain sex chromosome genetic conditions, such as Klinefelter's syndrome, will have a higher level of estradiol.[18]
Skeletal system
Estradiol has a profound effect on bone. Individuals without it (or other estrogens) will become tall and eunuchoid, as epiphyseal closure is delayed or may not take place.[19] Bone density is also affected, resulting in early osteopenia and osteoporosis.[20] Low levels of estradiol may also predict fractures, with post-menopausal women having the highest incidence of bone fracture.[21] Women past menopause experience an accelerated loss of bone mass due to a relative estrogen deficiency.[22]
Skin health
The estrogen receptor, as well as the progesterone receptor, have been detected in the skin, including in keratinocytes and fibroblasts.[23][24] At menopause and thereafter, decreased levels of female sex hormones result in atrophy, thinning, and increased wrinkling of the skin and a reduction in skin elasticity, firmness, and strength.[23][24] These skin changes constitute an acceleration in skin aging and are the result of decreased collagen content, irregularities in the morphology of epidermal skin cells, decreased ground substance between skin fibers, and reduced capillaries and blood flow.[23][24] The skin also becomes more dry during menopause, which is due to reduced skin hydration and surface lipids (sebum production).[23] Along with chronological aging and photoaging, estrogen deficiency in menopause is one of the three main factors that predominantly influences skin aging.[23]
Hormone replacement therapy consisting of systemic treatment with estrogen alone or in combination with a progestogen, has well-documented and considerable beneficial effects on the skin of postmenopausal women.[23][24] These benefits include increased skin collagen content, skin thickness and elasticity, and skin hydration and surface lipids.[23][24] Topical estrogen has been found to have similar beneficial effects on the skin.[23] In addition, a study has found that topical 2% progesterone cream significantly increases skin elasticity and firmness and observably decreases wrinkles in peri- and postmenopausal women.[24] Skin hydration and surface lipids, on the other hand, did not significantly change with topical progesterone.[24] These findings suggest that progesterone, like estrogen, also has beneficial effects on the skin, and may be independently protective against skin aging.[24]
Nervous system
Estrogens can be produced in the brain from steroid precursors. As antioxidants, they have been found to have neuroprotective function.[25]
The positive and negative feedback loops of the menstrual cycle involve ovarian estradiol as the link to the hypothalamic-pituitary system to regulate gonadotropins.[26]
Estrogen is considered to play a significant role in women's mental health, with links suggested between the hormone level, mood and well-being. Sudden drops or fluctuations in, or long periods of sustained low levels of estrogen may be correlated with significant mood-lowering. Clinical recovery from depression postpartum, perimenopause, and postmenopause was shown to be effective after levels of estrogen were stabilized and/or restored.[27][28]
The volumes of sexually dimorphic brain structures in transgender women were found to change and approximate typical female brain structures when exposed to estrogen concomitantly with androgen deprivation over a period of months,[29] suggesting that estrogen and/or androgens have a significant part to play in sex differentiation of the brain, both prenatally and later in life.
There is also evidence the programming of adult male sexual behavior in many vertebrates is largely dependent on estradiol produced during prenatal life and early infancy.[30] It is not yet known whether this process plays a significant role in human sexual behavior, although evidence from other mammals tends to indicate a connection.[31]
Estrogen has been found to increase the secretion of oxytocin and to increase the expression of its receptor, the oxytocin receptor, in the brain.[32] In women, a single dose of estradiol has been found to be sufficient to increase circulating oxytocin concentrations.[33]
Gynecological cancers
Estradiol has been tied to the development and progression of cancers such as breast cancer, ovarian cancer and endometrial cancer. Estradiol affects target tissues mainly by interacting with two nuclear receptors called estrogen receptor α (ERα) and estrogen receptor β (ERβ).[34][35] One of the functions of these estrogen receptors is the modulation of gene expression. Once estradiol binds to the ERs, the receptor complexes then bind to specific DNA sequences, possibly causing damage to the DNA and an increase in cell division and DNA replication. Eukaryotic cells respond to damaged DNA by stimulating or impairing G1, S, or G2 phases of the cell cycle to initiate DNA repair. As a result, cellular transformation and cancer cell proliferation occurs.[36]
Cardiovascular system
Estrogen affects certain blood vessels. Improvement in arterial blood flow has been demonstrated in coronary arteries.[37] 17-beta-estradiol (E2) is considered the most potent estrogen found in humans. E2 influences vascular function, apoptosis, and damage during cardiac ischemia and reperfusion. E2 can protect the heart and individual cardiac myocytes from injuries related to ischemia. After a heart attack or long periods of hypertension, E2 inhibits the adverse effects of pathologic remodeling of the heart.[38]
During pregnancy, high levels of estrogens, namely estradiol, increase coagulation and the risk of venous thromboembolism.
| Absolute incidence of first VTE per 10,000 person–years during pregnancy and the postpartum period | ||||||||
|---|---|---|---|---|---|---|---|---|
| Swedish data A | Swedish data B | English data | Danish data | |||||
| Time period | N | Rate (95% CI) | N | Rate (95% CI) | N | Rate (95% CI) | N | Rate (95% CI) |
| Outside pregnancy | 1105 | 4.2 (4.0–4.4) | 1015 | 3.8 (?) | 1480 | 3.2 (3.0–3.3) | 2895 | 3.6 (3.4–3.7) |
| Antepartum | 995 | 20.5 (19.2–21.8) | 690 | 14.2 (13.2–15.3) | 156 | 9.9 (8.5–11.6) | 491 | 10.7 (9.7–11.6) |
| Trimester 1 | 207 | 13.6 (11.8–15.5) | 172 | 11.3 (9.7–13.1) | 23 | 4.6 (3.1–7.0) | 61 | 4.1 (3.2–5.2) |
| Trimester 2 | 275 | 17.4 (15.4–19.6) | 178 | 11.2 (9.7–13.0) | 30 | 5.8 (4.1–8.3) | 75 | 5.7 (4.6–7.2) |
| Trimester 3 | 513 | 29.2 (26.8–31.9) | 340 | 19.4 (17.4–21.6) | 103 | 18.2 (15.0–22.1) | 355 | 19.7 (17.7–21.9) |
| Around delivery | 115 | 154.6 (128.8–185.6) | 79 | 106.1 (85.1–132.3) | 34 | 142.8 (102.0–199.8) | –
| |
| Postpartum | 649 | 42.3 (39.2–45.7) | 509 | 33.1 (30.4–36.1) | 135 | 27.4 (23.1–32.4) | 218 | 17.5 (15.3–20.0) |
| Early postpartum | 584 | 75.4 (69.6–81.8) | 460 | 59.3 (54.1–65.0) | 177 | 46.8 (39.1–56.1) | 199 | 30.4 (26.4–35.0) |
| Late postpartum | 65 | 8.5 (7.0–10.9) | 49 | 6.4 (4.9–8.5) | 18 | 7.3 (4.6–11.6) | 319 | 3.2 (1.9–5.0) |
| Incidence rate ratios (IRRs) of first VTE during pregnancy and the postpartum period | ||||||||
| Swedish data A | Swedish data B | English data | Danish data | |||||
| Time period | IRR* (95% CI) | IRR* (95% CI) | IRR (95% CI)† | IRR (95% CI)† | ||||
| Outside pregnancy | Reference (i.e., 1.00)
| |||||||
| Antepartum | 5.08 (4.66–5.54) | 3.80 (3.44–4.19) | 3.10 (2.63–3.66) | 2.95 (2.68–3.25) | ||||
| Trimester 1 | 3.42 (2.95–3.98) | 3.04 (2.58–3.56) | 1.46 (0.96–2.20) | 1.12 (0.86–1.45) | ||||
| Trimester 2 | 4.31 (3.78–4.93) | 3.01 (2.56–3.53) | 1.82 (1.27–2.62) | 1.58 (1.24–1.99) | ||||
| Trimester 3 | 7.14 (6.43–7.94) | 5.12 (4.53–5.80) | 5.69 (4.66–6.95) | 5.48 (4.89–6.12) | ||||
| Around delivery | 37.5 (30.9–44.45) | 27.97 (22.24–35.17) | 44.5 (31.68–62.54) | –
| ||||
| Postpartum | 10.21 (9.27–11.25) | 8.72 (7.83–9.70) | 8.54 (7.16–10.19) | 4.85 (4.21–5.57) | ||||
| Early postpartum | 19.27 (16.53–20.21) | 15.62 (14.00–17.45) | 14.61 (12.10–17.67) | 8.44 (7.27–9.75) | ||||
| Late postpartum | 2.06 (1.60–2.64) | 1.69 (1.26–2.25) | 2.29 (1.44–3.65) | 0.89 (0.53–1.39) | ||||
| Notes: Swedish data A = Using any code for VTE regardless of confirmation. Swedish data B = Using only algorithm-confirmed VTE. Early postpartum = First 6 weeks after delivery. Late postpartum = More than 6 weeks after delivery. * = Adjusted for age and calendar year. † = Unadjusted ratio calculated based on the data provided. Source: [39] | ||||||||
Other functions
Estradiol has complex effects on the liver. It affects the production of multiple proteins, including lipoproteins, binding proteins, and proteins responsible for blood clotting.[citation needed] In high amounts, estradiol can lead to cholestasis, for instance cholestasis of pregnancy.
Certain gynecological conditions are dependent on estrogen, such as endometriosis, leiomyomata uteri, and uterine bleeding.[citation needed]
Biological activity
Estradiol acts primarily as an agonist of the estrogen receptor (ER), a nuclear steroid hormone receptor. There are two subtypes of the ER, ERα and ERβ, and estradiol potently binds to and activates both of these receptors. The result of ER activation is a modulation of gene transcription and expression in ER-expressing cells, which is the predominant mechanism by which estradiol mediates its biological effects in the body. Estradiol also acts as an agonist of membrane estrogen receptors (mERs), such as GPER (GPR30), a recently discovered non-nuclear receptor for estradiol, via which it can mediate a variety of rapid, non-genomic effects.[40] Unlike the case of the ER, GPER appears to be selective for estradiol, and shows very low affinities for other endogenous estrogens, such as estrone and estriol.[41] Additional mERs besides GPER include ER-X, ERx, and Gq-mER.[42][43]
ERα/ERβ are in inactive state trapped in multimolecular chaperone complexes organized around the heat shock protein 90 (HSP90), containing p23 protein, and immunophilin, and located in majority in cytoplasm and partially in nucleus. In the E2 classical pathway or estrogen classical pathway, estradiol enters the cytoplasm, where it interacts with ERs. Once bound E2, ERs dissociate from the molecular chaperone complexes and become competent to dimerize, migrate to nucleus, and to bind to specific DNA sequences (estrogen response element, ERE), allowing for gene transcription which can take place over hours and days.
Given by subcutaneous injection in mice, estradiol is about 10-fold more potent than estrone and about 100-fold more potent than estriol.[44][45][46] As such, estradiol is the main estrogen in the body, although the roles of estrone and estriol as estrogens are said not to be negligible.[46]
| Estrogen | ER RBA (%) | Uterine weight (%) | Uterotrophy | LH levels (%) | SHBG RBA (%) |
|---|---|---|---|---|---|
| Control | – | 100 | – | 100 | – |
| Estradiol (E2) | 100 | 506 ± 20 | +++ | 12–19 | 100 |
| Estrone (E1) | 11 ± 8 | 490 ± 22 | +++ | ? | 20 |
| Estriol (E3) | 10 ± 4 | 468 ± 30 | +++ | 8–18 | 3 |
| Estetrol (E4) | 0.5 ± 0.2 | ? | Inactive | ? | 1 |
| 17α-Estradiol | 4.2 ± 0.8 | ? | ? | ? | ? |
| 2-Hydroxyestradiol | 24 ± 7 | 285 ± 8 | +b | 31–61 | 28 |
| 2-Methoxyestradiol | 0.05 ± 0.04 | 101 | Inactive | ? | 130 |
| 4-Hydroxyestradiol | 45 ± 12 | ? | ? | ? | ? |
| 4-Methoxyestradiol | 1.3 ± 0.2 | 260 | ++ | ? | 9 |
| 4-Fluoroestradiola | 180 ± 43 | ? | +++ | ? | ? |
| 2-Hydroxyestrone | 1.9 ± 0.8 | 130 ± 9 | Inactive | 110–142 | 8 |
| 2-Methoxyestrone | 0.01 ± 0.00 | 103 ± 7 | Inactive | 95–100 | 120 |
| 4-Hydroxyestrone | 11 ± 4 | 351 | ++ | 21–50 | 35 |
| 4-Methoxyestrone | 0.13 ± 0.04 | 338 | ++ | 65–92 | 12 |
| 16α-Hydroxyestrone | 2.8 ± 1.0 | 552 ± 42 | +++ | 7–24 | <0.5 |
| 2-Hydroxyestriol | 0.9 ± 0.3 | 302 | +b | ? | ? |
| 2-Methoxyestriol | 0.01 ± 0.00 | ? | Inactive | ? | 4 |
| Notes: Values are mean ± SD or range. ER RBA = Relative binding affinity to estrogen receptors of rat uterine cytosol. Uterine weight = Percentage change in uterine wet weight of ovariectomized rats after 72 hours with continuous administration of 1 μg/hour via subcutaneously implanted osmotic pumps. LH levels = Luteinizing hormone levels relative to baseline of ovariectomized rats after 24 to 72 hours of continuous administration via subcutaneous implant. Footnotes: a = Synthetic (i.e., not endogenous). b = Atypical uterotrophic effect which plateaus within 48 hours (estradiol's uterotrophy continues linearly up to 72 hours). Sources: See template. | |||||
Biochemistry
Biosynthesis
Estradiol, like other steroid hormones, is derived from cholesterol. After side chain cleavage and using the Δ5 or the Δ4- pathway, androstenedione is the key intermediary. A portion of the androstenedione is converted to testosterone, which in turn undergoes conversion to estradiol by aromatase. In an alternative pathway, androstenedione is aromatized to estrone, which is subsequently converted to estradiol via 17β-hydroxysteroid dehydrogenase (17β-HSD).[48]
During the reproductive years, most estradiol in women is produced by the granulosa cells of the ovaries by the aromatization of androstenedione (produced in the theca folliculi cells) to estrone, followed by conversion of estrone to estradiol by 17β-HSD. Smaller amounts of estradiol are also produced by the adrenal cortex, and, in men, by the testes.テンプレート:Medcn
Estradiol is not produced in the gonads only; in particular, fat cells produce active precursors to estradiol, and will continue to do so even after menopause.[49] Estradiol is also produced in the brain and in arterial walls.
In men, approximately 15 to 25% of circulating estradiol is produced in the testicles.[50][51] The rest is synthesized via peripheral aromatization of testosterone into estradiol and of androstenedione into estrone (which is then transformed into estradiol via peripheral 17β-HSD).[50][51] This peripheral aromatization occurs predominantly in adipose tissue, but also occurs in other tissues such as bone, liver, and the brain.[50] Approximately 40 to 50 μg of estradiol is produced per day in men.[50]
Distribution
In plasma, estradiol is largely bound to SHBG and albumin. Only about 2.21% (± 0.04%) of estradiol is free and biologically active. The percentage remains constant throughout the menstrual cycle.[52]
Metabolism
Metabolic pathways of estradiol in humans
|
Inactivation of estradiol includes conversion to less-active estrogens, such as estrone and estriol. Estriol is the major urinary metabolite.[citation needed] Estradiol is conjugated in the liver to form estrogen conjugates like estradiol sulfate, estradiol glucuronide and, as such, excreted via the kidneys. Some of the water-soluble conjugates are excreted via the bile duct, and partly reabsorbed after hydrolysis from the intestinal tract. This enterohepatic circulation contributes to maintaining estradiol levels.
Estradiol is also metabolized via hydroxylation into catechol estrogens. In the liver, it is non-specifically metabolized by CYP1A2, CYP3A4, and CYP2C9 via 2-hydroxylation into 2-hydroxyestradiol, and by CYP2C9, CYP2C19, and CYP2C8 via 17β-hydroxy dehydrogenation into estrone,[53] with various other cytochrome P450 (CYP) enzymes and metabolic transformations also being involved.[54]
Estradiol is additionally conjugated with an ester into lipoidal estradiol forms like estradiol palmitate and estradiol stearate to a certain extent; these esters are stored in adipose tissue and may act as a very long-lasting reservoir of estradiol.[55][56]
Excretion
Estradiol is excreted in the form of glucuronide and sulfate estrogen conjugates in urine. Following an intravenous injection of labeled estradiol in women, almost 90% is excreted in urine and feces within 4 to 5 days.[57][58] Enterohepatic recirculation causes a delay in excretion of estradiol.[57]
Levels
Levels of estradiol in premenopausal women are highly variable throughout the menstrual cycle and reference ranges widely vary from source to source.[60] Estradiol levels are minimal and according to most laboratories range from 20 to 80 pg/mL during the early to mid follicular phase (or the first week of the menstrual cycle, also known as menses).[61][62] Levels of estradiol gradually increase during this time and through the mid to late follicular phase (or the second week of the menstrual cycle) until the pre-ovulatory phase.[60][61] At the time of pre-ovulation (a period of about 24 to 48 hours), estradiol levels briefly surge and reach their highest concentrations of any other time during the menstrual cycle.[60] Circulating levels are typically between 130 and 200 pg/mL at this time, but in some women may be as high as 300 to 400 pg/mL, and the upper limit of the reference range of some laboratories are even greater (for instance, 750 pg/mL).[60][61][63][64][65] Following ovulation (or mid-cycle) and during the latter half of the menstrual cycle or the luteal phase, estradiol levels plateau and fluctuate between around 100 and 150 pg/mL during the early and mid luteal phase, and at the time of the late luteal phase, or a few days before menstruation, reach a low of around 40 pg/mL.[60][62] The mean integrated levels of estradiol during a full menstrual cycle have variously been reported by different sources as 80, 120, and 150 pg/mL.[62][66][67] Although contradictory reports exist, one study found mean integrated estradiol levels of 150 pg/mL in younger women whereas mean integrated levels ranged from 50 to 120 pg/mL in older women.[67]
During the reproductive years of human females, levels of estradiol are somewhat higher than that of estrone, except during the early follicular phase of the menstrual cycle; thus, estradiol may be considered the predominant estrogen during human female reproductive years in terms of absolute serum levels and estrogenic activity.[citation needed] During pregnancy, estriol becomes the predominant circulating estrogen, and this is the only time at which estetrol occurs in the body, while during menopause, estrone predominates (both based on serum levels).[citation needed] The estradiol produced by male humans, from testosterone, is present at serum levels roughly comparable to those of postmenopausal women (14–55 versus <35 pg/mL, respectively).[citation needed] It has also been reported that if concentrations of estradiol in a 70-year-old man are compared to those of a 70-year-old woman, levels are approximately 2- to 4-fold higher in the man.[68]
| Group | E2 (prod) | E2 (levels) | E1 (levels) | Ratio |
|---|---|---|---|---|
| Pubertal girlsa Tanner stage I (childhood) Tanner stage II (ages 8–12) Tanner stage III (ages 10–13) Tanner stage IV (ages 11–14) Tanner stage V (ages 12–15) Follicular (days 1–14) Luteal (days 15–28) |
? ? ? ? ? ? |
9 (<9–20) pg/mL 15 (<9–30) pg/mL 27 (<9–60) pg/mL 55 (16–85) pg/mL 50 (30–100) pg/mL 130 (70–300) pg/mL |
13 (<9–23) pg/mL 18 (10–37) pg/mL 26 (17–58) pg/mL 36 (23–69) pg/mL 44 (30–89) pg/mL 75 (39–160) pg/mL |
? ? ? ? ? ? |
| Prepubertal boys | ? | 2–8 pg/mL | ? | ? |
| Premenopausal women Early follicular phase (days 1–4) Mid follicular phase (days 5–9) Late follicular phase (days 10–14) Luteal phase (days 15–28) Oral contraceptive (anovulatory) |
30–100 μg/day 100–160 μg/day 320–640 μg/day 300 μg/day ? |
40–60 pg/mL 60–100 pg/mL 200–400 pg/mL 190 pg/mL 12–50 pg/mL |
40–60 pg/mL ? 170–200 pg/mL 100–150 pg/mL ? |
0.5–1 ? 1–2 1.5 ? |
| Postmenopausal women | 18 μg/day | 5–20 pg/mL | 30–70 pg/mL | 0.3–0.8 |
| Pregnant women First trimester (weeks 1–12) Second trimester (weeks 13–26) Third trimester (weeks 27–40) |
? ? ? |
1,000–5,000 pg/mL 5,000–15,000 pg/mL 10,000–40,000 pg/mL |
? ? ? |
? ? ? |
| Mena | 20–60 μg/day | 27 (20–55) pg/mL | 20–90 pg/mL | 0.4–0.6 |
| Footnotes: a = Format is "mean value (range)" or just "range". Sources: [69][70][71][72][73][59][74] | ||||
Measurement
In women, serum estradiol is measured in a clinical laboratory and reflects primarily the activity of the ovaries. The Estradiol blood test measures the amount of estradiol in the blood.[75] It is used to check the function of the ovaries, placenta, adrenal glands.[75] This can detect baseline estrogen in women with amenorrhea or menstrual dysfunction, and to detect the state of hypoestrogenicity and menopause. Furthermore, estrogen monitoring during fertility therapy assesses follicular growth and is useful in monitoring the treatment. Estrogen-producing tumors will demonstrate persistent high levels of estradiol and other estrogens. In precocious puberty, estradiol levels are inappropriately increased.
Ranges
Individual laboratory results should always be interpreted using the ranges provided by the laboratory that performed the test.
| Patient type | Lower limit | Upper limit | Unit |
|---|---|---|---|
| Adult male | 50[76] | 200[76] | pmol/L |
| 14 | 55 | pg/mL | |
| Adult female (follicular phase, day 5) |
70[76] 95% PI (standard) |
500[76] 95% PI |
pmol/L |
| 110[77] 90% PI (used in diagram) |
220[77] 90% PI | ||
| 19 (95% PI) | 140 (95% PI) | pg/mL | |
| 30 (90% PI) | 60 (90% PI) | ||
| Adult female (preovulatory peak) |
400[76] | 1500[76] | pmol/L |
| 110 | 410 | pg/mL | |
| Adult female (luteal phase) |
70[76] | 600[76] | pmol/L |
| 19 | 160 | pg/mL | |
| Adult female – free (not protein bound) |
0.5[78]テンプレート:Original research inline | 9[78]テンプレート:Original research inline | pg/mL |
| 1.7[78]テンプレート:Original research inline | 33[78]テンプレート:Original research inline | pmol/L | |
| Post-menopausal female | N/A[76] | < 130[76] | pmol/L |
| N/A | < 35 | pg/mL |
In the normal menstrual cycle, estradiol levels measure typically <50 pg/mL at menstruation, rise with follicular development (peak: 200 pg/mL), drop briefly at ovulation, and rise again during the luteal phase for a second peak. At the end of the luteal phase, estradiol levels drop to their menstrual levels unless there is a pregnancy.
During pregnancy, estrogen levels, including estradiol, rise steadily toward term. The source of these estrogens is the placenta, which aromatizes prohormones produced in the fetal adrenal gland.
Medical use
Estradiol is used as a medication, primarily in hormone therapy for menopausal symptoms as well as feminizing hormone therapy for trans individuals.[80]
Chemistry
Estradiol is an estrane steroid.[80] It is also known as 17β-estradiol (to distinguish it from 17α-estradiol) or as estra-1,3,5(10)-triene-3,17β-diol. It has two hydroxyl groups, one at the C3 position and the other at the 17β position, as well as three double bonds in the A ring. Due to its two hydroxyl groups, estradiol is often abbreviated as E2. The structurally related estrogens, estrone (E1), estriol (E3), and estetrol (E4) have one, three, and four hydroxyl groups, respectively.
Neuropsychopharmacology
Product insert information, accompanying commercial perscription estradiol, indicates it causes depression. In a randomized, double-blind, placebo-controlled study, estradiol was shown to have gender-specific effects on fairness sensitivity. Overall, when the division of a given amount of money was framed as either fair or unfair in a modified version of the ultimatum game, estradiol increased the acceptance rate of fair-framed proposals among men and decreased it among women. However, among the placebo-group "the mere belief of receiving estradiol treatment significantly increased the acceptance of unfair-framed offers in both sexes", indicating that so-called "environmental" factors played a role in organising the responses towards these presentations of the ultimatum game.[81]
History
The discovery of estrogen is usually credited to the American scientists Edgar Allen and Edward A. Doisy.[82][83] In 1923, they observed that injection of fluid from porcine ovarian follicles produced pubertal- and estrus-type changes (including vaginal, uterine, and mammary gland changes and sexual receptivity) in sexually immature, ovariectomized mice and rats.[82][83][84] These findings demonstrated the existence of a hormone which is produced by the ovaries and is involved in sexual maturation and reproduction.[82][83][84] At the time of its discovery, Allen and Doisy did not name the hormone, and simply referred to it as an "ovarian hormone" or "follicular hormone";[83] others referred to it variously as feminin, folliculin, menformon, thelykinin, and emmenin.[85][86] In 1926, Parkes and Bellerby coined the term estrin to describe the hormone on the basis of it inducing estrus in animals.[87][85] Estrone was isolated and purified independently by Allen and Doisy and German scientist Adolf Butenandt in 1929, and estriol was isolated and purified by Marrian in 1930; they were the first estrogens to be identified.[83][88][89]
Estradiol, the most potent of the three major estrogens, was the last of the three to be identified.[83][87] It was discovered by Schwenk and Hildebrant in 1933, who synthesized it via reduction of estrone.[83] Estradiol was subsequently isolated and purified from sow ovaries by Doisy in 1935, with its chemical structure determined simultaneously,[90] and was referred to variously as dihydrotheelin, dihydrofolliculin, dihydrofollicular hormone, and dihydroxyestrin.[83][91][92] In 1935, the name estradiol and the term estrogen were formally established by the Sex Hormone Committee of the Health Organization of the League of Nations; this followed the names estrone (which was initially called theelin, progynon, folliculin, and ketohydroxyestrin) and estriol (initially called theelol and trihydroxyestrin) having been established in 1932 at the first meeting of the International Conference on the Standardization of Sex Hormones in London.[87][93] Following its discovery, a partial synthesis of estradiol from cholesterol was developed by Inhoffen and Hohlweg in 1940, and a total synthesis was developed by Anner and Miescher in 1948.[83]
Society and culture
Etymology
The name estradiol derives from テンプレート:Not a typo, Gk. οἶστρος (テンプレート:Transl, literally meaning "verve or inspiration"),[94] which refers to the estrane steroid ring system, and テンプレート:Not a typo, a chemical term and suffix indicating that the compound is a type of alcohol bearing two hydroxyl groups.
References
- ↑ 以下の位置に戻る: 1.0 1.1 1.2 1.3 1.4 Stanczyk FZ, Archer DF, Bhavnani BR (6月 2013). "Ethinyl estradiol and 17β-estradiol in combined oral contraceptives: pharmacokinetics, pharmacodynamics and risk assessment". Contraception. 87 (6): 706–27. doi:10.1016/j.contraception.2012.12.011. PMID 23375353.
- ↑ Falcone T, Hurd WW (2007). Clinical Reproductive Medicine and Surgery. Elsevier Health Sciences. pp. 22–. ISBN 978-0-323-03309-1. Archived from the original on 10 1月 2023. Retrieved 22 10月 2016.
{{cite book}}:|archive-date=/|archive-url=timestamp mismatch; 10 1月 2023 suggested (help) - ↑ Price TM, Blauer KL, Hansen M, Stanczyk F, Lobo R, Bates GW (3月 1997). "Single-dose pharmacokinetics of sublingual versus oral administration of micronized 17 beta-estradiol". Obstetrics and Gynecology. 89 (3): 340–5. doi:10.1016/S0029-7844(96)00513-3. PMID 9052581. S2CID 71641652.
- ↑ Naunton M, Al Hadithy AF, Brouwers JR, Archer DF (2006). "Estradiol gel: review of the pharmacology, pharmacokinetics, efficacy, and safety in menopausal women". Menopause. 13 (3): 517–27. doi:10.1097/01.gme.0000191881.52175.8c. PMID 16735950. S2CID 42748448.
- ↑ Ryan KJ (8月 1982). "Biochemistry of aromatase: significance to female reproductive physiology". Cancer Research. 42 (8 Suppl): 3342s – 3344s. PMID 7083198.
- ↑ Mechoulam R, Brueggemeier RW, Denlinger DL (9月 1984). "Estrogens in insects". Cellular and Molecular Life Sciences. 40 (9): 942–944. doi:10.1007/BF01946450. S2CID 31950471.
- ↑ Ozon R (1972). "Estrogens in Fishes, Amphibians, Reptiles, and Birds". In Idler DR (ed.). Steroids In Nonmammalian Vertebrates. Oxford: Elsevier Science. pp. 390–414. ISBN 978-0-323-14098-0. Archived from the original on 10 1月 2023. Retrieved 17 10月 2020.
{{cite book}}:|archive-date=/|archive-url=timestamp mismatch; 10 1月 2023 suggested (help) - ↑ Saldanha, Colin J., Luke Remage-Healey, and Barney A. Schlinger. "Synaptocrine signaling: steroid synthesis and action at the synapse." Endocrine reviews 32.4 (2011): 532–549.
- ↑ 以下の位置に戻る: 9.0 9.1 9.2 McMillan JA, Feigin RD, DeAngelis C, Jones MD (2006). Oski's Pediatrics: Principles & Practice. Lippincott Williams & Wilkins. pp. 550–. ISBN 978-0-7817-3894-1.
- ↑ 以下の位置に戻る: 10.0 10.1 10.2 Craig CR, Stitzel RE (2004). Modern Pharmacology with Clinical Applications. Lippincott Williams & Wilkins. pp. 706–. ISBN 978-0-7817-3762-3.
- ↑ Preedy VR (2 12月 2011). Handbook of Growth and Growth Monitoring in Health and Disease. Springer Science & Business Media. pp. 2661–. ISBN 978-1-4419-1794-2. Archived from the original on 10 1月 2023. Retrieved 9 6月 2017.
{{cite book}}:|archive-date=/|archive-url=timestamp mismatch; 10 1月 2023 suggested (help) - ↑ Carreau S, Lambard S, Delalande C, Denis-Galeraud I, Bilinska B, Bourguiba S (4月 2003). "Aromatase expression and role of estrogens in male gonad : a review". Reproductive Biology and Endocrinology. 1: 35. doi:10.1186/1477-7827-1-35. PMC 155680. PMID 12747806.
- ↑ Pentikäinen V, Erkkilä K, Suomalainen L, Parvinen M, Dunkel L (5月 2000). "Estradiol acts as a germ cell survival factor in the human testis in vitro". The Journal of Clinical Endocrinology and Metabolism. 85 (5): 2057–67. doi:10.1210/jcem.85.5.6600. PMID 10843196.
- ↑ Sharpe RM, Skakkebaek NE (5月 1993). "Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract?". Lancet. 341 (8857): 1392–5. doi:10.1016/0140-6736(93)90953-E. PMID 8098802. S2CID 33135527.
- ↑ Handelsman DJ (2001). "Estrogens and falling sperm counts". Reproduction, Fertility, and Development. 13 (4): 317–24. doi:10.1071/rd00103. PMID 11800170.
- ↑ Fisch H, Goldstedin R (2003). "Environmental estrogens and sperm counts" (PDF). Pure and Applied Chemistry. 75 (11–12): 2181–2193. doi:10.1351/pac200375112181. S2CID 11068097. Archived from the original (PDF) on 4 3月 2016. Retrieved 29 12月 2015.
{{cite journal}}:|archive-date=/|archive-url=timestamp mismatch; 4 3月 2016 suggested (help) - ↑ Raman JD, Schlegel PN (2月 2002). "Aromatase inhibitors for male infertility". The Journal of Urology. 167 (2 Pt 1): 624–9. doi:10.1016/S0022-5347(01)69099-2. PMID 11792932.
- ↑ Visootsak J, Graham JM (10月 2006). "Klinefelter syndrome and other sex chromosomal aneuploidies". Orphanet Journal of Rare Diseases. 1 (42): 42. doi:10.1186/1750-1172-1-42. PMC 1634840. PMID 17062147.
- ↑ Vanderschueren D, Laurent MR, Claessens F, Gielen E, Lagerquist MK, Vandenput L, et al. (12月 2014). "Sex steroid actions in male bone". Endocrine Reviews. 35 (6): 906–60. doi:10.1210/er.2014-1024. PMC 4234776. PMID 25202834.
- ↑ Carani C, Qin K, Simoni M, Faustini-Fustini M, Serpente S, Boyd J, et al. (7月 1997). "Effect of testosterone and estradiol in a man with aromatase deficiency". The New England Journal of Medicine. 337 (2): 91–5. doi:10.1056/NEJM199707103370204. PMID 9211678.
- ↑ Bergh C, Wennergren D, Möller M, Brisby H (21 12月 2020). "Fracture incidence in adults in relation to age and gender: A study of 27,169 fractures in the Swedish Fracture Register in a well-defined catchment area". PLOS ONE. 15 (12): e0244291. Bibcode:2020PLoSO..1544291B. doi:10.1371/journal.pone.0244291. PMC 7751975. PMID 33347485.
- ↑ Albright F, Smith PH, Richardson AM (31 5月 1941). "Postmenopausal Osteoporosis: Its Clinical Features". JAMA. 116 (22): 2465–2474. doi:10.1001/jama.1941.02820220007002.
- ↑ 以下の位置に戻る: 23.0 23.1 23.2 23.3 23.4 23.5 23.6 23.7 Raine-Fenning NJ, Brincat MP, Muscat-Baron Y (2003). "Skin aging and menopause : implications for treatment". American Journal of Clinical Dermatology. 4 (6): 371–8. doi:10.2165/00128071-200304060-00001. PMID 12762829. S2CID 20392538.
- ↑ 以下の位置に戻る: 24.0 24.1 24.2 24.3 24.4 24.5 24.6 24.7 Holzer G, Riegler E, Hönigsmann H, Farokhnia S, Schmidt JB, Schmidt B (9月 2005). "Effects and side-effects of 2% progesterone cream on the skin of peri- and postmenopausal women: results from a double-blind, vehicle-controlled, randomized study". The British Journal of Dermatology. 153 (3): 626–34. doi:10.1111/j.1365-2133.2005.06685.x. PMID 16120154. S2CID 6077829.
- ↑ Behl C, Widmann M, Trapp T, Holsboer F (11月 1995). "17-beta estradiol protects neurons from oxidative stress-induced cell death in vitro". Biochemical and Biophysical Research Communications. 216 (2): 473–82. doi:10.1006/bbrc.1995.2647. PMID 7488136.
- ↑ Meethal SV, Liu T, Chan HW, Ginsburg E, Wilson AC, Gray DN, Bowen RL, Vonderhaar BK, Atwood CS (8月 2009). "Identification of a regulatory loop for the synthesis of neurosteroids: a steroidogenic acute regulatory protein-dependent mechanism involving hypothalamic-pituitary-gonadal axis receptors". Journal of Neurochemistry. 110 (3): 1014–27. doi:10.1111/j.1471-4159.2009.06192.x. PMC 2789665. PMID 19493163.
- ↑ Douma SL, Husband C, O'Donnell ME, Barwin BN, Woodend AK (2005). "Estrogen-related mood disorders: reproductive life cycle factors". Advances in Nursing Science. 28 (4): 364–75. doi:10.1097/00012272-200510000-00008. PMID 16292022. S2CID 9172877.
- ↑ Lasiuk GC, Hegadoren KM (10月 2007). "The effects of estradiol on central serotonergic systems and its relationship to mood in women". Biological Research for Nursing. 9 (2): 147–60. doi:10.1177/1099800407305600. PMID 17909167. S2CID 37965502.
- ↑ Hulshoff HE, Cohen-Kettenis PT, Van Haren NE, Peper JS, Brans RG, Cahn W, Schnack HG, Gooren LJ, Kahn RS (7月 2006). "Changing your sex changes your brain: influences of testosterone and estrogen on adult human brain structure". European Journal of Endocrinology. 155 (suppl_1): 107–114. doi:10.1530/eje.1.02248.
- ↑ Harding CF (6月 2004). "Hormonal modulation of singing: hormonal modulation of the songbird brain and singing behavior". Annals of the New York Academy of Sciences. 1016 (1): 524–39. Bibcode:2004NYASA1016..524H. doi:10.1196/annals.1298.030. PMID 15313793. S2CID 12457330. Archived from the original on 27 9月 2007.
{{cite journal}}:|archive-date=/|archive-url=timestamp mismatch; 27 9月 2007 suggested (help) - ↑ Simerly RB (27 3月 2002). "Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain" (PDF). Annual Review of Neuroscience. 25: 507–36. doi:10.1146/annurev.neuro.25.112701.142745. PMID 12052919. Archived from the original (PDF) on 1 10月 2008. Retrieved 7 3月 2007.
{{cite journal}}:|archive-date=/|archive-url=timestamp mismatch; 1 10月 2008 suggested (help) - ↑ Goldstein I, Meston CM, Davis S, Traish A (17 11月 2005). Women's Sexual Function and Dysfunction: Study, Diagnosis and Treatment. CRC Press. pp. 205–. ISBN 978-1-84214-263-9.
- ↑ Acevedo-Rodriguez A, Mani SK, Handa RJ (2015). "Oxytocin and Estrogen Receptor β in the Brain: An Overview". Frontiers in Endocrinology. 6: 160. doi:10.3389/fendo.2015.00160. PMC 4606117. PMID 26528239.
- ↑ Bulzomi P, Bolli A, Galluzzo P, Leone S, Acconcia F, Marino M (1月 2010). "Naringenin and 17beta-estradiol coadministration prevents hormone-induced human cancer cell growth". IUBMB Life. 62 (1): 51–60. doi:10.1002/iub.279. PMID 19960539. S2CID 7903757.
- ↑ Sreeja S, Santhosh Kumar TR, Lakshmi BS, Sreeja S (7月 2012). "Pomegranate extract demonstrate a selective estrogen receptor modulator profile in human tumor cell lines and in vivo models of estrogen deprivation". The Journal of Nutritional Biochemistry. 23 (7): 725–32. doi:10.1016/j.jnutbio.2011.03.015. PMID 21839626.
- ↑ Thomas CG, Strom A, Lindberg K, Gustafsson JA (6月 2011). "Estrogen receptor beta decreases survival of p53-defective cancer cells after DNA damage by impairing G₂/M checkpoint signaling". Breast Cancer Research and Treatment. 127 (2): 417–27. doi:10.1007/s10549-010-1011-z. PMID 20623183. S2CID 6752694.
- ↑ Collins P, Rosano GM, Sarrel PM, Ulrich L, Adamopoulos S, Beale CM, McNeill JG, Poole-Wilson PA (7月 1995). "17 beta-Estradiol attenuates acetylcholine-induced coronary arterial constriction in women but not men with coronary heart disease". Circulation. 92 (1): 24–30. doi:10.1161/01.CIR.92.1.24. PMID 7788912.
- ↑ Knowlton, A.A.; Lee, A.R. (7月 2012). "Estrogen and the cardiovascular system". Pharmacology & Therapeutics. 135 (1): 54–70. doi:10.1016/j.pharmthera.2012.03.007. PMC 5688223. PMID 22484805.
- ↑ Abdul Sultan A, West J, Stephansson O, Grainge MJ, Tata LJ, Fleming KM, Humes D, Ludvigsson JF (11月 2015). "Defining venous thromboembolism and measuring its incidence using Swedish health registries: a nationwide pregnancy cohort study". BMJ Open. 5 (11): e008864. doi:10.1136/bmjopen-2015-008864. PMC 4654387. PMID 26560059.
- ↑ Prossnitz ER, Barton M (5月 2014). "Estrogen biology: new insights into GPER function and clinical opportunities". Molecular and Cellular Endocrinology. 389 (1–2): 71–83. doi:10.1016/j.mce.2014.02.002. PMC 4040308. PMID 24530924.
- ↑ Prossnitz ER, Arterburn JB, Sklar LA (2007). "GPR30: A G protein-coupled receptor for estrogen". Mol. Cell. Endocrinol. 265–266: 138–42. doi:10.1016/j.mce.2006.12.010. PMC 1847610. PMID 17222505.
- ↑ Soltysik K, Czekaj P (4月 2013). "Membrane estrogen receptors – is it an alternative way of estrogen action?". Journal of Physiology and Pharmacology. 64 (2): 129–42. PMID 23756388.
- ↑ Micevych PE, Kelly MJ (2012). "Membrane estrogen receptor regulation of hypothalamic function". Neuroendocrinology. 96 (2): 103–10. doi:10.1159/000338400. PMC 3496782. PMID 22538318.
- ↑ Labhart A (6 12月 2012). Clinical Endocrinology: Theory and Practice. Springer Science & Business Media. pp. 548–. ISBN 978-3-642-96158-8. Archived from the original on 10 1月 2023. Retrieved 11 11月 2018.
{{cite book}}:|archive-date=/|archive-url=timestamp mismatch; 10 1月 2023 suggested (help) - ↑ Tucker SB (2007). Maternal, Fetal, & Neonatal Physiology: A Clinical Perspective. Elsevier Health Sciences. pp. 43–. ISBN 978-1-4160-2944-1. Archived from the original on 10 1月 2023. Retrieved 7 6月 2017.
{{cite book}}:|archive-date=/|archive-url=timestamp mismatch; 10 1月 2023 suggested (help) - ↑ 以下の位置に戻る: 46.0 46.1 Hall JE (31 5月 2015). Guyton and Hall Textbook of Medical Physiology E-Book. Elsevier Health Sciences. pp. 1043–. ISBN 978-0-323-38930-3.
- ↑ Häggström M, Richfield D (2014). "Diagram of the pathways of human steroidogenesis". WikiJournal of Medicine. 1 (1). doi:10.15347/wjm/2014.005. ISSN 2002-4436.
- ↑ Boron WF, Boulpaep EL (2003). Medical Physiology: A Cellular And Molecular Approach. Elsevier/Saunders. p. 1300. ISBN 978-1-4160-2328-9.
- ↑ Mutschler E, Schäfer-Korting M (2001). Arzneimittelwirkungen (in Deutsch) (8 ed.). Stuttgart: Wissenschaftliche Verlagsgesellschaft. pp. 434, 444. ISBN 978-3-8047-1763-3.
- ↑ 以下の位置に戻る: 50.0 50.1 50.2 50.3 Melmed S (1 1月 2016). Williams Textbook of Endocrinology. Elsevier Health Sciences. pp. 710–. ISBN 978-0-323-29738-7. Archived from the original on 10 1月 2023. Retrieved 21 3月 2018.
{{cite book}}:|archive-date=/|archive-url=timestamp mismatch; 10 1月 2023 suggested (help) - ↑ 以下の位置に戻る: 51.0 51.1 Marcus R, Feldman D, Dempster DW, Luckey M, Cauley JA (13 6月 2013). Osteoporosis. Academic Press. pp. 331–. ISBN 978-0-12-398252-0.
- ↑ Wu CH, Motohashi T, Abdel-Rahman HA, Flickinger GL, Mikhail G (8月 1976). "Free and protein-bound plasma estradiol-17 beta during the menstrual cycle". The Journal of Clinical Endocrinology and Metabolism. 43 (2): 436–45. doi:10.1210/jcem-43-2-436. PMID 950372.
- ↑ Cheng ZN, Shu Y, Liu ZQ, Wang LS, Ou-Yang DS, Zhou HH (2月 2001). "Role of cytochrome P450 in estradiol metabolism in vitro". Acta Pharmacologica Sinica. 22 (2): 148–54. PMID 11741520.
- ↑ Lee AJ, Cai MX, Thomas PE, Conney AH, Zhu BT (8月 2003). "Characterization of the oxidative metabolites of 17beta-estradiol and estrone formed by 15 selectively expressed human cytochrome p450 isoforms". Endocrinology. 144 (8): 3382–98. doi:10.1210/en.2003-0192. PMID 12865317.
- ↑ Oettel M, Schillinger E (6 12月 2012). Estrogens and Antiestrogens I: Physiology and Mechanisms of Action of Estrogens and Antiestrogens. Springer Science & Business Media. pp. 235–237. ISBN 978-3-642-58616-3.
- ↑ Oettel M, Schillinger E (6 12月 2012). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. pp. 268, 271. ISBN 978-3-642-60107-1.
- ↑ 以下の位置に戻る: 57.0 57.1 Dorfman, Ralph I. (1961). "Steroid Hormone Metabolism". Radioactive Isotopes in Physiology Diagnostics and Therapy / Künstliche Radioaktive Isotope in Physiologie Diagnostik und Therapie. pp. 1223–1241. doi:10.1007/978-3-642-49761-2_39. ISBN 978-3-642-49477-2.
- ↑ Sandberg AA, Slaunwhite WR (8月 1957). "Studies on phenolic steroids in human subjects. II. The metabolic fate and hepato-biliary-enteric circulation of C14-estrone and C14-estradiol in women". J. Clin. Invest. 36 (8): 1266–78. doi:10.1172/JCI103524. PMC 1072719. PMID 13463090.
- ↑ 以下の位置に戻る: 59.0 59.1 "Estradiol" (PDF). ilexmedical.com. Archived (PDF) from the original on 4 2月 2024. Retrieved 4 7月 2024.
{{cite web}}:|archive-date=/|archive-url=timestamp mismatch; 4 2月 2024 suggested (help) - ↑ 以下の位置に戻る: 60.0 60.1 60.2 60.3 60.4 Becker JB, Berkley KJ, Geary N, Hampson E, Herman JP, Young E (4 12月 2007). Sex Differences in the Brain: From Genes to Behavior. Oxford University Press. pp. 64–. ISBN 978-0-19-804255-6.
Estradiol levels are minimal during the earliest days of the follicular phase, but increasing concentrations are released into the general circulation as the follicle matures. The highest levels are reached about 24 to 48 hours before the LH peak. In fact, the pre-ovulatory peak in estradiol represents its highest concentration during the entire menstrual cycle. Serum concentrations at this time are typically about 130–200 pg/mL, but concentrations as high as 300–400 pg/mL can be achieved in some women. Following a transient fall in association with ovulation, estradiol secretion is restored by production from the corpus luteum during the luteal phase. Plateau levels of around 100–150 pg/mL (Abraham, 1978; Thorneycroft et al., 1971) are most often seen during the period from −10 to −5 days before the onset of menses. With the regression of the corpus luteum, estradiol levels fall, gradually in some women and precipitously in others, during the last few days of the luteal phase. This ushers in the onset of menses, the sloughing of the endometrium. Serum estradiol during menses is approximately 30–50 pg/mL. (Source.)
- ↑ 以下の位置に戻る: 61.0 61.1 61.2 Strauss JR, Barbieri RL (2009). Yen and Jaffe's Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. Elsevier Health Sciences. pp. 807–. ISBN 978-1-4160-4907-4. Archived from the original on 10 1月 2023. Retrieved 21 12月 2016.
In most laboratories, serum estradiol levels range from 20 to 80 pg/mL during the early to midfollicular phase of the menstrual cycle and peak at 200 to 500 pg/mL during the preovulatory surge. During the midluteal phase, serum estradiol levels range from 60 to 200 pg/mL.
{{cite book}}:|archive-date=/|archive-url=timestamp mismatch; 10 1月 2023 suggested (help) - ↑ 以下の位置に戻る: 62.0 62.1 62.2 Christian C, von Schoultz B (15 3月 1994). Hormone Replacement Therapy: Standardized or Individually Adapted Doses?. CRC Press. pp. 60–. ISBN 978-1-85070-545-1.
Plasma levels of estradiol range from 40 to 80 pg/mL during the 1st week of the ovarian cycle (early follicular phase) and from 80 to 300 pg/mL during the 2nd week (mid- and late follicular phase including periovulatory peak). Then during the 3rd and 4th weeks, estradiol fluctuates between 100 and 150 pg/mL (early and mid-luteal phase) to 40 pg/mL a few days before menstruation (late luteal phase). The mean integrated estradiol level during a full 28-day normal cycle is around 80 pg/mL.
- ↑ Jameson JL, De Groot LJ (18 5月 2010). Endocrinology: Adult and Pediatric. Elsevier Health Sciences. pp. 2812–. ISBN 978-1-4557-1126-0.
Midcycle: 150-750 pg/mL
- ↑ Hay ID, Wass JA (26 1月 2009). Clinical Endocrine Oncology. John Wiley & Sons. pp. 623–. ISBN 978-1-4443-0023-9.
Mid-cycle: 110-330 pg/mL
- ↑ Dons RF (12 7月 1994). Endocrine and Metabolic Testing Manual. CRC Press. pp. 8–. ISBN 978-0-8493-7657-3.
Ovulatory: 200-400 pg/mL
- ↑ Notelovitz M, van Keep PA (6 12月 2012). The Climacteric in Perspective: Proceedings of the Fourth International Congress on the Menopause, held at Lake Buena Vista, Florida, October 28 – November 2, 1984. Springer Science & Business Media. pp. 397–. ISBN 978-94-009-4145-8. Archived from the original on 10 1月 2023. Retrieved 22 10月 2016.
[...] following the menopause, circulating estradiol levels decrease from a premenopausal mean of 120 pg/mL to only 13 pg/mL.
{{cite book}}:|archive-date=/|archive-url=timestamp mismatch; 10 1月 2023 suggested (help) - ↑ 以下の位置に戻る: 67.0 67.1 Müller EE, MacLeod RM (6 12月 2012). Neuroendocrine Perspectives. Springer Science & Business Media. pp. 121–. ISBN 978-1-4612-3554-5. Archived from the original on 10 1月 2023. Retrieved 22 10月 2016.
[...] [premenopausal] mean [estradiol] concentration of 150 pg/mL [...]
{{cite book}}:|archive-date=/|archive-url=timestamp mismatch; 10 1月 2023 suggested (help) - ↑ Sayed Y, Taxel P (12月 2003). "The use of estrogen therapy in men". Current Opinion in Pharmacology. 3 (6): 650–4. doi:10.1016/j.coph.2003.07.004. PMID 14644018.
- ↑ Nichols KC, Schenkel L, Benson H (1984). "17 beta-estradiol for postmenopausal estrogen replacement therapy". Obstet Gynecol Surv. 39 (4): 230–45. doi:10.1097/00006254-198404000-00022. PMID 6717863.
- ↑ Cynthia C. Chernecky; Barbara J. Berger (31 10月 2012). Laboratory Tests and Diagnostic Procedures – E-Book. Elsevier Health Sciences. pp. 488–. ISBN 978-1-4557-4502-9. Archived from the original on 22 8月 2023. Retrieved 22 8月 2023.
{{cite book}}:|archive-date=/|archive-url=timestamp mismatch; 22 8月 2023 suggested (help) - ↑ Powers MS, Schenkel L, Darley PE, Good WR, Balestra JC, Place VA (8月 1985). "Pharmacokinetics and pharmacodynamics of transdermal dosage forms of 17 beta-estradiol: comparison with conventional oral estrogens used for hormone replacement". Am. J. Obstet. Gynecol. 152 (8): 1099–106. doi:10.1016/0002-9378(85)90569-1. PMID 2992279.
- ↑ Kenneth L. Becker (2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. pp. 889, 1059–1060, 2153. ISBN 978-0-7817-1750-2.
- ↑ Lalit Bajaj; Stephen Berman (1 1月 2011). Berman's Pediatric Decision Making. Elsevier Health Sciences. pp. 160–. ISBN 978-0-323-05405-8. Archived from the original on 11 1月 2023. Retrieved 22 8月 2023.
{{cite book}}:|archive-date=/|archive-url=timestamp mismatch; 11 1月 2023 suggested (help) - ↑ Kuhl H (2003). "Östrogene für den Mann?" [Estrogens for the man?]. Blickpunkt der Mann. 1 (3): 6–12. ISSN 1727-0669. Archived from the original on 22 8月 2023. Retrieved 22 8月 2023.
{{cite journal}}:|archive-date=/|archive-url=timestamp mismatch; 22 8月 2023 suggested (help) - ↑ 以下の位置に戻る: 75.0 75.1 "Estradiol blood test: MedlinePlus Medical Encyclopedia". medlineplus.gov (in English). Archived from the original on 18 3月 2021. Retrieved 6 5月 2019.
{{cite web}}:|archive-date=/|archive-url=timestamp mismatch; 18 3月 2021 suggested (help) - ↑ 以下の位置に戻る: 76.0 76.1 76.2 76.3 76.4 76.5 76.6 76.7 76.8 76.9 GPNotebook — reference range (oestradiol) Archived 9 6月 2012 at the Wayback Machine Retrieved on 27 September 2009
- ↑ 以下の位置に戻る: 77.0 77.1 Values taken from day 1 after LH surge in: Stricker R, Eberhart R, Chevailler MC, Quinn FA, Bischof P, Stricker R (2006). "Establishment of detailed reference values for luteinizing hormone, follicle stimulating hormone, estradiol, and progesterone during different phases of the menstrual cycle on the Abbott ARCHITECT analyzer". Clinical Chemistry and Laboratory Medicine. 44 (7): 883–7. doi:10.1515/CCLM.2006.160. PMID 16776638. S2CID 524952. as PDF
- ↑ 以下の位置に戻る: 78.0 78.1 78.2 78.3 Total amount multiplied by 0.022 according to 2.2% presented in: Wu CH, Motohashi T, Abdel-Rahman HA, Flickinger GL, Mikhail G (8月 1976). "Free and protein-bound plasma estradiol-17 beta during the menstrual cycle". The Journal of Clinical Endocrinology and Metabolism. 43 (2): 436–45. doi:10.1210/jcem-43-2-436. PMID 950372.テンプレート:Original research inline
- ↑ Häggström M (2014). "Reference ranges for estradiol, progesterone, luteinizing hormone and follicle-stimulating hormone during the menstrual cycle". WikiJournal of Medicine. 1 (1). doi:10.15347/wjm/2014.001. ISSN 2002-4436.
- ↑ 以下の位置に戻る: 80.0 80.1 Kuhl H (8月 2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration". Climacteric. 8 (1 Suppl 1): 3–63. doi:10.1080/13697130500148875. PMID 16112947. S2CID 24616324.
- ↑ Coenjaerts M, Pape F, Santoso V, Grau F, Stoffel-Wagner B, Philipsen A, Schultz J, Hurlemann R, Scheele D (9月 2021). "Sex differences in economic decision-making: Exogenous estradiol has opposing effects on fairness framing in women and men". Eur. Neuropsychopharmacol. 50 (2): 46–54. doi:10.1016/j.euroneuro.2021.04.006. hdl:20.500.11811/11066. ISSN 0924-977X. PMID 33957337. S2CID 233982738.
- ↑ 以下の位置に戻る: 82.0 82.1 82.2 Loriaux DL, Loriaux L (14 3月 2016). A Biographical History of Endocrinology. John Wiley & Sons. pp. 345–. ISBN 978-1-119-20246-2.
- ↑ 以下の位置に戻る: 83.0 83.1 83.2 83.3 83.4 83.5 83.6 83.7 83.8 Lauritzen C, Studd JW (22 6月 2005). Current Management of the Menopause. CRC Press. pp. 44–. ISBN 978-0-203-48612-2.
- ↑ 以下の位置に戻る: 84.0 84.1 Allen E, Doisy EA (1923). "An Ovarian Hormone". Journal of the American Medical Association. 81 (10): 819. doi:10.1001/jama.1923.02650100027012. ISSN 0002-9955.
- ↑ 以下の位置に戻る: 85.0 85.1 Gruhn JG, Kazer RR (11 11月 2013). Hormonal Regulation of the Menstrual Cycle: The Evolution of Concepts. Springer Science & Business Media. pp. 69–73. ISBN 978-1-4899-3496-3.
- ↑ Newerla GJ (1944). "The History of the Discovery and Isolation of the Female Sex Hormones". New England Journal of Medicine. 230 (20): 595–604. doi:10.1056/NEJM194405182302001. ISSN 0028-4793.
- ↑ 以下の位置に戻る: 87.0 87.1 87.2 Fritz MA, Speroff L (28 3月 2012). Clinical Gynecologic Endocrinology and Infertility. Lippincott Williams & Wilkins. pp. 750–. ISBN 978-1-4511-4847-3.
- ↑ Parl FF (2000). Estrogens, Estrogen Receptor and Breast Cancer. IOS Press. pp. 4–. ISBN 978-0-9673355-4-4. Archived from the original on 10 1月 2023. Retrieved 27 11月 2016.
{{cite book}}:|archive-date=/|archive-url=timestamp mismatch; 10 1月 2023 suggested (help) - ↑ Sartorelli AC, Johns DG (27 11月 2013). Antineoplastic and Immunosuppressive Agents. Springer Science & Business Media. pp. 104–. ISBN 978-3-642-65806-8.
- ↑ Shoupe D, Haseltine FP (6 12月 2012). Contraception. Springer Science & Business Media. pp. 2–. ISBN 978-1-4612-2730-4.
- ↑ MacCorquodale DW, Thayer SA, Doisy EA (1935). "The Crystalline Ovarian Follicular Hormone". Experimental Biology and Medicine. 32 (7): 1182. doi:10.3181/00379727-32-8020P. ISSN 1535-3702. S2CID 83557813.
- ↑ Chemicals Identified in Human Biological Media: A Data Base. Design and Development Branch, Survey and Analysis Division, Office of Program Integration and Information, Office of Pesticides and Toxic Substances, Environmental Protection Agency. 1981. pp. 114–.
- ↑ Fausto-Sterling A (2000). Sexing the Body: Gender Politics and the Construction of Sexuality. Basic Books. pp. 189–. ISBN 978-0-465-07714-4.
- ↑ "Greek Word Study Tool: oistros". Perseus Digital Library. Archived from the original on 17 3月 2012. Retrieved 28 12月 2011.
{{cite web}}:|archive-date=/|archive-url=timestamp mismatch; 17 3月 2012 suggested (help)
引用エラー: <references> で定義されている name "FordRoach2013" の <ref> タグは、先行するテキスト内で使用されていません。
<references> で定義されている name "HochadelMosby2015" の <ref> タグは、先行するテキスト内で使用されていません。
| ||
| ||
- テンプレート呼び出しで引数が重複しているページ
- 参照エラーのあるページ
- CS1 errors: archive-url
- CS1: long volume value
- CS1 Deutsch-language sources (de)
- CS1 English-language sources (en)
- Webarchive template wayback links
- Use dmy dates from August 2018
- Articles with invalid date parameter in template
- 壊れたファイルへのリンクがあるページ
- Chem-molar-mass both hardcoded and calculated
- Pages using Chem molar mass with unsupported parameters
- All articles with unsourced statements
- Articles with unsourced statements from February 2013
- Articles with hatnote templates targeting a nonexistent page
- Articles with unsourced statements from December 2016
- Articles with unsourced statements from June 2014
- Articles containing Ancient Greek (to 1453)-language text
- Estradiol
- Secondary alcohols
- Animal reproductive system
- Antigonadotropins
- Antioxidants
- Estranes
- Estrogens
- GPER agonists
- Hepatotoxins
- Hormones of the hypothalamus-pituitary-gonad axis
- Hormones of the hypothalamic-pituitary-prolactin axis
- Hormones of the pregnant female
- Human female endocrine system
- Hydroxyarenes
- Prolactin releasers
- Sex hormones
