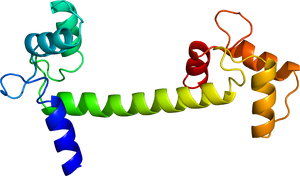
カルモジュリン
藤井 哉
東京大学医学系研究科神経生化学教室
DOI:10.14931/bsd.4597 原稿受付日:2015年8月7日 原稿完成日:2015年9月30日
担当編集委員:和田 圭司(国立研究開発法人国立精神・神経医療研究センター)
英:calmodulin
カルモジュリンは148アミノ酸残基、分子量約16.7kDa、酸性のCa2++結合タンパク質であり、それぞれ2つのEFハンドドメインからなるN末側ドメインとC末側ドメインがリンカーでつながったダンベル様構造をしている。カルモジュリンは、酵母、植物、昆虫からヒトまで真核生物に発現しており、特に脊椎動物の中では高い保存性を示す。Ca2+と結合することで、Ca2+バッファーとして働くほか、下流のタンパク質に結合して活性などを調節し、Ca2+センサーとしてCa2+シグナル伝達の中でも非常に重要な役割を果たす。特に脳においては、Ca2+シグナル伝達をコントロールする中心的な役割を担い、神経突起形成、軸索伸展、シナプス形成、シナプス可塑性、記憶・学習など様々な機能に関わる。
| カルモジュリン | |||||||||
|---|---|---|---|---|---|---|---|---|---|
カルモジュリンEF-handの結晶構造[1] | |||||||||
| Identifiers | |||||||||
| Symbol | efhand | ||||||||
| Pfam | PF00036 | ||||||||
| InterPro | IPR002048 | ||||||||
| PROSITE | PDOC00018 | ||||||||
| SCOP | 1osa | ||||||||
| SUPERFAMILY | 1osa | ||||||||
| OPM protein | 1djx | ||||||||
| CDD | cd00051 | ||||||||
| |||||||||
| Calmodulin 2 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||
| Identifiers | |||||||||||||
| Symbols | CALM2; CAMII; LQT15; PHKD; PHKD2; caM | ||||||||||||
| External IDs | OMIM: 114182 MGI: 103250 HomoloGene: 134804 GeneCards: CALM2 Gene | ||||||||||||
| EC number | 2.7.11.19 | ||||||||||||
| |||||||||||||
| Orthologs | |||||||||||||
| Species | Human | Mouse | |||||||||||
| Entrez | 805 | 640703 | |||||||||||
| Ensembl | ENSG00000143933 | ENSMUSG00000036438 | |||||||||||
| UniProt | P62158 | P62204 | |||||||||||
| RefSeq (mRNA) | NM_001305624 | NM_007589 | |||||||||||
| RefSeq (protein) | NP_001292553 | NP_031615 | |||||||||||
| Location (UCSC) |
Chr 2: 47.16 – 47.18 Mb |
Chr 17: 87.83 – 87.85 Mb | |||||||||||
| PubMed search | [3] | [4] | |||||||||||
| Calmodulin 3 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||
| Identifiers | |||||||||||||
| Symbols | CALM3; HEL-S-72; PHKD; PHKD3 | ||||||||||||
| External IDs | OMIM: 114183 MGI: 103249 HomoloGene: 134804 GeneCards: CALM3 Gene | ||||||||||||
| EC number | 2.7.11.19 | ||||||||||||
| |||||||||||||
| Orthologs | |||||||||||||
| Species | Human | Mouse | |||||||||||
| Entrez | 808 | 640703 | |||||||||||
| Ensembl | ENSG00000160014 | ENSMUSG00000019370 | |||||||||||
| UniProt | P62158 | P62204 | |||||||||||
| RefSeq (mRNA) | NM_005184 | NM_007590 | |||||||||||
| RefSeq (protein) | NP_005175 | NP_031616 | |||||||||||
| Location (UCSC) |
Chr 19: 46.6 – 46.61 Mb |
Chr 7: 17.5 – 17.51 Mb | |||||||||||
| PubMed search | [5] | [6] | |||||||||||
発見
1970年、Kakiuchiらは、ラット脳抽出物中の環状ヌクレオチドフォスフォジエステラーゼ活性がCa2+により制御されることを報告し[2]、このCa2+依存性を担う調節因子を見出した[3][4]。また、同じ1970年に独立してCheungは環状ヌクレオチドフォスフォジエステラーゼの活性が精製の過程で減弱することから、精製の過程で分離される分画より活性化因子を発見し報告した[5]。
1973年にTeoとWangらはウシの心臓からこの活性化因子を精製し[6]、これらの別々に発見された因子の正体が同一のCa2+結合タンパク質であることを示した[7][8]。
その後、トロポニンCに特性が類似したタンパク質であることが示され[9] [10]、アミノ酸配列が決定され[11]、分光学的解析によってCa2+結合に伴って構造が変化することが示された[12] [13] [14]。その呼び名は研究グループによって、activator protein, modulator protein, Ca2+dependent regulator protein(CDR)、Phosphodiesterase Activating Factor(PAF)などさまざまに呼ばれたが、1970年代末にcalmodulinという名称が付けられた[15][16]。
構造
カルモジュリンは148残基のアミノ酸からなる、分子量約16.7kDaのタンパク質である。1985年にCa2+存在下のウシ由来カルモジュリンのX線結晶構造が解かれ、原子レベルでの構造が明らかになった[17]。Ca2+と結合する4つのヘリックス・ループ・ヘリックス構造のEFハンドモチーフを持ち、2つずつがそれぞれペアとなって球状のN末側ドメイン、C末側ドメインを形成し、その間をリンカーがつながったダンベル様の構造をしている。それぞれの球状のドメインの大きさは約25×20×20 Åであり、分子全体としては長軸が約65 Åの長さである[17]。
サブファミリー
ヒトのCalmodulin1、Calmodulin2、Calmodulin3は同一のアミノ酸配列のタンパク質をコードしており、それぞれ染色体上の14q24-q31、2p21.1-p21.3、19q13.2-q13.3に位置する(表1)[18]。
| タンパク質名称 | NCBI遺伝子情報 | NCBI mRNA情報 (RefSeq) | HUGO遺伝子命名法委員会 (HGNC) | Allen mouse brain |
|---|---|---|---|---|
| Calmodulin1 | 801 | NM_006888 | CALM1 | 12098 |
| Calmodulin2 | 805 | NM_001743 | CALM2 | 12099 |
| Calmodulin3 | 808 | NM_005184 | CALM3 | 12100 |
機能
カルモジュリンは脳内で10~100 µmol/lの濃度で発現しており[19]、細胞内で上昇したCa2+と結合し、Ca2+バッファーとして働くのに加え、様々なカルモジュリン結合タンパク質と結合して生理機能を発揮する(表2)。
エフェクタータンパク質
カルモジュリンの主要な機能は、細胞内のCa2+濃度の変化を感知し、カルモジュリン結合タンパク質の機能制御を通じて、細胞機能を制御(活性化、抑制)することであり、その具体的な効果はターゲットとなる下流のタンパク質によって様々に異なる。多くはCa2+依存性がありCa2+/カルモジュリンと結合するが、Ca2+と結合していないカルモジュリンと結合するタンパク質や、Ca2+非依存的に結合するタンパク質も存在する。
Ca2+に対する親和性の違いから、C末側ドメインはN末側ドメインに比べCa2+に対する親和性が高く、in vitroでトリプシン処理により得られたN末側/C末側ドメインのCa2+親和性をpH 7.5, 100 mM KCl, 25 ℃の条件下で測定した場合には、それぞれ1.5~100 μM、0.4~10 μMである[20]。Ca2+依存的な結合の場合、カルモジュリンがCa2+と結合することで、疎水性領域が露出し、ターゲットとなるタンパク質のカルモジュリン結合ドメインにある疎水性のアミノ酸残基と相互作用する。この疎水性アミノ酸残基の位置によって、幾つかのもシーフに分類される[21][22][23]。
- 1-14モチーフ:(ミオシン軽鎖キナーゼ(myosin light-chain kinase, MLCK)、カルシニューリン、Ca2+/カルモジュリン依存性タンパク質キナーゼIV(CaMKIV)、一酸化窒素合成酵素(NOS))
- 1-10モチーフ:(Ca2+/カルモジュリン依存性タンパク質キナーゼII(CaMKII)、シナプシン、熱ショックタンパク質70/90)
- 1-16モチーフ:(Ca2+/カルモジュリン依存性タンパク質キナーゼキナーゼ, CaMKK)
- IQモチーフ:Ca2+非依存的な結合タンパク質に多い。コンセンサス配列はIQXXXRGXXXRである。
また、カルモジュリンはリン酸化[24]や糖化[25]、メチル化[11]など翻訳後修飾を受け、機能を調節することが知られている[26][27]。
| タンパク質名称 | 参考文献 | |
|---|---|---|
| 環状ヌクレオチド代謝酵素 | ホスホジエステラーゼ | [2] |
| アデニル酸シクラーゼ | [28][29][30] | |
| 膜タンパク質 | 細胞膜カルシウムATPアーゼ (plasma membrane Ca2+-ATPase; PMCA) | [31][32][33] |
| NMDA型グルタミン酸受容体 | [34] | |
| 代謝活性型グルタミン酸受容体 | [35][36] | |
| L型カルシウムチャネル | [37][38] | |
| P/Q型カルシウムチャネル | [39] | |
| IP3受容体 | [40]) | |
| リン酸化酵素 | ミオシン軽鎖キナーゼ (MLCK) | [41][42][43][44] |
| Ca2+/カルモジュリン依存性タンパク質キナーゼI (CaMKI) | [45] | |
| Ca2+/カルモジュリン依存性タンパク質キナーゼII (CaMKII) | [46][47] | |
| Ca2+/カルモジュリン依存性タンパク質キナーゼIV (CaMKIV) | [48] | |
| Ca2+/カルモジュリン依存性タンパク質キナーゼキナーゼ (CaMKK) | [49][50] | |
| ホスホリラーゼキナーゼ | [51]) | |
| 脱リン酸化酵素 | カルシニューリン | [52][53] |
| 細胞骨格系タンパク質 | MAP2 | [54] |
| タウ | [54] | |
| アデューシン | [55] | |
| ミオシン | [56][57][58][59] | |
| 一酸化窒素合成酵素 | [60][61] | |
| 熱ショックタンパク質70/90 | [62][63] | |
神経系での機能
神経回路の発達
神経突起形成[64][65]、軸索伸展[66][67][68]、シナプスの形成[69]などを通して、神経回路の発達に関わる。
例えば、発生期に神経細胞が軸索を伸展し標的となる細胞に投射して神経回路を構築する際には、軸索の先端部は成長円錐を形成し、細胞外の軸索ガイダンス分子などのシグナルに応じて誘引されたり反発されたりすることで、その伸展する方向を制御している。アフリカツメガエルの脊髄神経細胞やニワトリの後根神経節細胞を用いた実験などから、ガイダンス分子としてネトリン1[70][71]やSEMA3A[72][73]をはじめさまざま知られており、これらは受容体を介して局所的なCa2+上昇を引き起こし、その濃度や局在によってカルモジュリンは異なるターゲットを活性化し、成長円錐の誘引や反発をコントロールしている。
シナプス可塑性、記憶・学習
脳機能において、カルモジュリンは、そのターゲットとなるCaMKII、カルシニューリン、アデニル酸シクラーゼなどの下流のエフェクター酵素の制御を通してのシナプス可塑性や記憶・学習の制御に関して不可欠な役割を果たしている。
海馬CA1領域における長期増強や長期抑圧はNMDA型グルタミン酸受容体の活性化によりCa2+が流入し、カルモジュリンと結合することで下流の酵素を活性化して引き起こされる。中でも、カルモジュリンの脳内での主要なターゲットのひとつであるCaMKIIは、Ca2+濃度の低い基底状態ではカルモジュリン結合ドメインとオーバーラップしている自己抑制ドメインによってそのキナーゼ活性が低く抑えられているが、Ca2+上昇に伴ってCa2+/カルモジュリンと結合し、コンフォメーションが変化することでこの自己抑制がはずれ、活性化する[74]。また、CaMKIIは12量体を作っており[75][76]、活性化に伴って隣接するキナーゼサブユニットの間で286番目のスレオニンがリン酸化することで、Ca2+/カルモジュリンとの親和性が高くなるとともに[77]、Ca2+/カルモジュリンが解離した後も部分的な活性を持続する"Autonomous"な状態を保持することができる[78][79]。CaMKIIは海馬のシェーファー側枝からCA1錐体細胞への長期増強に関わることがが報告されており[80][81][82]、CaMKIIαのノックアウトマウスや点変異導入マウスでは海馬依存的な空間学習に異常がみられる[83][84]。 同様にカルモジュリンによって活性化されるアデニル酸シクラーゼ1、8やカルシニューリンもシナプス可塑性や記憶・学習に関与することが薬理学的実験や遺伝子改変動物実験などによって報告されている[85][86][87][88]。
こうした電気生理学的・行動学的な変化を引き起こす分子・細胞生物学的なプロセスとして、カルモジュリンはCa2+流入に伴うスパインの構造的可塑性の誘導[89][90][91]やアクチン細胞骨格の再構築[92][93]、種々の酵素の活性化[94][95][96]やCREBを介した新規遺伝子発現[97][98][99]に関わることが示されている。また、数あるカルモジュリン依存的な酵素の活性化は均等に起こるのではなく、Ca2+流入に伴うカルモジュリン依存的な酵素の活性化は均等に起こるのではなく、神経入力のパターンに応じて上昇したCa2+の時間的・空間的拡がりに応じて異なる強弱で活性化され、状況に応じて適切な神経細胞機能を発現していると考えられている[100][101]。
阻害剤
1974年にWeissらが、カルモジュリンにより活性化される脳のホスホジエステラーゼに対するフェノチアジン誘導体の阻害効果の作用機序およびキネティクスを報告し、カルモジュリン阻害剤であることを示した[102](これに先立つ1968年、Hondaらはフェノチアジン誘導体の環状ヌクレオチドホスホジエステラーゼに対する阻害効果が脳由来の酵素と心臓由来の酵素で異なることを報告している[103])。この後、W-7[104]やカルミダゾリウム[105]など、さまざまな物質がカルモジュリン阻害剤として働くことが見出されている[106][107]。
疾患と関連するカルモジュリンの変異
カルモジュリンの点突然変異が、カテコールアミン誘発性多形性心室性頻拍、QT延長症候群、特発性心室細動で見出されている(表3)。
| 疾患名 | 遺伝子名 | 変異 | 文献 |
|---|---|---|---|
| カテコールアミン誘発性多形性心室性頻拍 (CPVT) | CALM1 | N53I | [108] |
| N97S | [108] | ||
| QT延長症候群 (LQTS) | CALM1 | D129G | [109] |
| F141L | [109] | ||
| CALM2 | D95V | [110] | |
| N97S | [110] | ||
| N97I | [109] | ||
| D133H | [110] | ||
| CLAM3 | D129G | [111] | |
| CPVT・LQTSの合併 | CALM2 | D131E | [110] |
| Q135P | [110] | ||
| 特発性心室細動(IVF) | CALM1 | F89L | [112] |
また、癌ゲノム解析により、多数の体細胞変異が見つかっているが、その機能については良く分かっていない[113][114][115]。
カルモジュリンを用いたCa2+インディケーター
カルモジュリンがCa2+依存的にターゲットペプチドと相互作用することを用いて、様々なGenetically-encoded Ca2+ indicatorが開発されている。大まかには、2色の異なる色の蛍光タンパク質間の蛍光共鳴エネルギー移動を用いてその2色の蛍光強度の比をレシオメトリック測定することが可能なFRETセンサー(Cameleonなど)と[116][117]、円順列変異GFPを用いてその蛍光強度からCa2+濃度を測定する緑色蛍光プローブ(G-CaMPなど)がある[118][119]。Cameleonの場合、Ca2+と結合したカルモジュリンがそのターゲットのM13ペプチドと結合することでコンフォメーションが変化し、2色の蛍光タンパク質の間での蛍光共鳴エネルギー移動の効率が変わることを利用している。一方で、G-CaMPの場合には、カルモジュリンとM13ペプチドの結合によるコンフォメーション変化が発色団周囲の環境を変化させることにより、蛍光強度が変化することを利用している。
2000年代以降、これらの改良が進んでおり、変化率を大きくしたものや単一活動電位を記録できる高感度のもの、キネティクスが速いもの、さまざまな色のインディケーターなどが開発され、生きた動物個体の中での神経細胞やシナプスの活動を長期間観察するのに用いられている[120][121][122][123][124][125][126][127][128][129]。
関連項目
参考文献
- ↑
Babu, Y.S., Bugg, C.E., & Cook, W.J. (1988).
Structure of calmodulin refined at 2.2 A resolution. Journal of molecular biology, 204(1), 191-204. [PubMed:3145979] [WorldCat] [DOI] - ↑ 2.0 2.1 S Kakiuchi, R Yamazaki
Stimulation of the activity of cyclic 3',5'-nucleotide phosphodiesterase by calcium ion.
Proc. Japan Acad. 46, 387-392:1970 - ↑ S Kakiuchi, R Yamazaki, H Nakajima
Properties of a heat-stable phosphodiesterase activating factor isolated from brain extract
Proc. Japan Acad. 46, 587-592:1970 - ↑
Kakiuchi, S., & Yamazaki, R. (1970).
Calcium dependent phosphodiesterase activity and its activating factor (PAF) from brain studies on cyclic 3',5'-nucleotide phosphodiesterase (3). Biochemical and biophysical research communications, 41(5), 1104-10. [PubMed:4320714] [WorldCat] [DOI] - ↑
Cheung, W.Y. (1970).
Cyclic 3',5'-nucleotide phosphodiesterase. Demonstration of an activator. Biochemical and biophysical research communications, 38(3), 533-8. [PubMed:4315350] [WorldCat] [DOI] - ↑
Teo, T.S., Wang, T.H., & Wang, J.H. (1973).
Purification and properties of the protein activator of bovine heart cyclic adenosine 3',5'-monophosphate phosphodiesterase. The Journal of biological chemistry, 248(2), 588-95. [PubMed:4346337] [WorldCat] - ↑
Teo, T.S., & Wang, J.H. (1973).
Mechanism of activation of a cyclic adenosine 3':5'-monophosphate phosphodiesterase from bovine heart by calcium ions. Identification of the protein activator as a Ca2+ binding protein. The Journal of biological chemistry, 248(17), 5950-5. [PubMed:4353626] [WorldCat] - ↑ 日高弘義、垣内史朗 編
カルモデュリン―Ca2+受容蛋白質
1981 - ↑
Stevens, F.C., Walsh, M., Ho, H.C., Teo, T.S., & Wang, J.H. (1976).
Comparison of calcium-binding proteins. Bovine heart and brain protein activators of cyclic nucleotide phosphodiesterase and rabbit skeletal muscle troponin C. The Journal of biological chemistry, 251(15), 4495-500. [PubMed:181374] [WorldCat] - ↑
Watterson, D.M., Harrelson, W.G., Keller, P.M., Sharief, F., & Vanaman, T.C. (1976).
Structural similarities between the Ca2+-dependent regulatory proteins of 3':5'-cyclic nucleotide phosphodiesterase and actomyosin ATPase. The Journal of biological chemistry, 251(15), 4501-13. [PubMed:181375] [WorldCat] - ↑ 11.0 11.1
Watterson, D.M., Sharief, F., & Vanaman, T.C. (1980).
The complete amino acid sequence of the Ca2+-dependent modulator protein (calmodulin) of bovine brain. The Journal of biological chemistry, 255(3), 962-75. [PubMed:7356670] [WorldCat] - ↑
Klee, C.B. (1977).
Conformational transition accompanying the binding of Ca2+ to the protein activator of 3',5'-cyclic adenosine monophosphate phosphodiesterase. Biochemistry, 16(5), 1017-24. [PubMed:14663] [WorldCat] [DOI] - ↑
Wolff, D.J., Poirier, P.G., Brostrom, C.O., & Brostrom, M.A. (1977).
Divalent cation binding properties of bovine brain Ca2+-dependent regulator protein. The Journal of biological chemistry, 252(12), 4108-17. [PubMed:193856] [WorldCat] - ↑
Dedman, J.R., Potter, J.D., Jackson, R.L., Johnson, J.D., & Means, A.R. (1977).
Physicochemical properties of rat testis Ca2+-dependent regulator protein of cyclic nucleotide phosphodiesterase. Relationship of Ca2+-binding, conformational changes, and phosphodiesterase activity. The Journal of biological chemistry, 252(23), 8415-22. [PubMed:200611] [WorldCat] - ↑ WY Cheung ed.
Calcium and Cell Function: Volume 1 Calmodulin
1980 Academic Press, New York, ISBN 1483204030 - ↑
Cheung, W.Y., Lynch, T.J., & Wallace, R.W. (1978).
An endogenous Ca2+-dependent activator protein of brain adenylate cyclase and cyclic neucleotide phosphodiesterase. Advances in cyclic nucleotide research, 9, 233-51. [PubMed:208377] [WorldCat] - ↑ 17.0 17.1
Babu, Y.S., Sack, J.S., Greenhough, T.J., Bugg, C.E., Means, A.R., & Cook, W.J. (1985).
Three-dimensional structure of calmodulin. Nature, 315(6014), 37-40. [PubMed:3990807] [WorldCat] [DOI] - ↑
Berchtold, M.W., Egli, R., Rhyner, J.A., Hameister, H., & Strehler, E.E. (1993).
Localization of the human bona fide calmodulin genes CALM1, CALM2, and CALM3 to chromosomes 14q24-q31, 2p21.1-p21.3, and 19q13.2-q13.3. Genomics, 16(2), 461-5. [PubMed:8314583] [WorldCat] [DOI] - ↑
Xia, Z., & Storm, D.R. (2005).
The role of calmodulin as a signal integrator for synaptic plasticity. Nature reviews. Neuroscience, 6(4), 267-76. [PubMed:15803158] [WorldCat] [DOI] - ↑
Linse, S., Helmersson, A., & Forsén, S. (1991).
Calcium binding to calmodulin and its globular domains. The Journal of biological chemistry, 266(13), 8050-4. [PubMed:1902469] [WorldCat] - ↑
Rhoads, A.R., & Friedberg, F. (1997).
Sequence motifs for calmodulin recognition. FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 11(5), 331-40. [PubMed:9141499] [WorldCat] [DOI] - ↑
Tidow, H., & Nissen, P. (2013).
Structural diversity of calmodulin binding to its target sites. The FEBS journal, 280(21), 5551-65. [PubMed:23601118] [WorldCat] [DOI] - ↑
Marshall, C.B., Nishikawa, T., Osawa, M., Stathopulos, P.B., & Ikura, M. (2015).
Calmodulin and STIM proteins: Two major calcium sensors in the cytoplasm and endoplasmic reticulum. Biochemical and biophysical research communications, 460(1), 5-21. [PubMed:25998729] [WorldCat] [DOI] - ↑
Plancke, Y.D., & Lazarides, E. (1983).
Evidence for a phosphorylated form of calmodulin in chicken brain and muscle. Molecular and cellular biology, 3(8), 1412-20. [PubMed:6621532] [PMC] [WorldCat] [DOI] - ↑
Kowluru, R.A., Heidorn, D.B., Edmondson, S.P., Bitensky, M.W., Kowluru, A., Downer, N.W., ..., & Trewhella, J. (1989).
Glycation of calmodulin: chemistry and structural and functional consequences. Biochemistry, 28(5), 2220-8. [PubMed:2541779] [WorldCat] [DOI] - ↑
Sacks, D.B., Davis, H.W., Williams, J.P., Sheehan, E.L., Garcia, J.G., & McDonald, J.M. (1992).
Phosphorylation by casein kinase II alters the biological activity of calmodulin. The Biochemical journal, 283 ( Pt 1), 21-4. [PubMed:1314563] [PMC] [WorldCat] [DOI] - ↑
Quadroni, M., L'Hostis, E.L., Corti, C., Myagkikh, I., Durussel, I., Cox, J., ..., & Carafoli, E. (1998).
Phosphorylation of calmodulin alters its potency as an activator of target enzymes. Biochemistry, 37(18), 6523-32. [PubMed:9572870] [WorldCat] [DOI] - ↑
Westcott, K.R., La Porte, D.C., & Storm, D.R. (1979).
Resolution of adenylate cyclase sensitive and insensitive to Ca2+ and calcium-dependent regulatory protein (CDR) by CDR-sepharose affinity chromatography. Proceedings of the National Academy of Sciences of the United States of America, 76(1), 204-8. [PubMed:284333] [PMC] [WorldCat] [DOI] - ↑
Krupinski, J., Coussen, F., Bakalyar, H.A., Tang, W.J., Feinstein, P.G., Orth, K., ..., & Gilman, A.G. (1989).
Adenylyl cyclase amino acid sequence: possible channel- or transporter-like structure. Science (New York, N.Y.), 244(4912), 1558-64. [PubMed:2472670] [WorldCat] [DOI] - ↑
Feinstein, P.G., Schrader, K.A., Bakalyar, H.A., Tang, W.J., Krupinski, J., Gilman, A.G., & Reed, R.R. (1991).
Molecular cloning and characterization of a Ca2+/calmodulin-insensitive adenylyl cyclase from rat brain. Proceedings of the National Academy of Sciences of the United States of America, 88(22), 10173-7. [PubMed:1719547] [PMC] [WorldCat] [DOI] - ↑
Gopinath, R.M., & Vincenzi, F.F. (1977).
Phosphodiesterase protein activator mimics red blood cell cytoplasmic activator of (Ca2+-Mg2+)ATPase. Biochemical and biophysical research communications, 77(4), 1203-9. [PubMed:197955] [WorldCat] [DOI] - ↑
Jarrett, H.W., & Penniston, J.T. (1977).
Partial purification of the Ca2+-Mg2+ ATPase activator from human erythrocytes: its similarity to the activator of 3':5' - cyclic nucleotide phosphodiesterase. Biochemical and biophysical research communications, 77(4), 1210-6. [PubMed:197956] [WorldCat] [DOI] - ↑
Vorherr, T., James, P., Krebs, J., Enyedi, A., McCormick, D.J., Penniston, J.T., & Carafoli, E. (1990).
Interaction of calmodulin with the calmodulin binding domain of the plasma membrane Ca2+ pump. Biochemistry, 29(2), 355-65. [PubMed:2154244] [WorldCat] [DOI] - ↑
Ehlers, M.D., Zhang, S., Bernhadt, J.P., & Huganir, R.L. (1996).
Inactivation of NMDA receptors by direct interaction of calmodulin with the NR1 subunit. Cell, 84(5), 745-55. [PubMed:8625412] [WorldCat] [DOI] - ↑
Minakami, R., Jinnai, N., & Sugiyama, H. (1997).
Phosphorylation and calmodulin binding of the metabotropic glutamate receptor subtype 5 (mGluR5) are antagonistic in vitro. The Journal of biological chemistry, 272(32), 20291-8. [PubMed:9242710] [WorldCat] [DOI] - ↑
Nakajima, Y., Yamamoto, T., Nakayama, T., & Nakanishi, S. (1999).
A relationship between protein kinase C phosphorylation and calmodulin binding to the metabotropic glutamate receptor subtype 7. The Journal of biological chemistry, 274(39), 27573-7. [PubMed:10488094] [WorldCat] [DOI] - ↑
Peterson, B.Z., DeMaria, C.D., Adelman, J.P., & Yue, D.T. (1999).
Calmodulin is the Ca2+ sensor for Ca2+ -dependent inactivation of L-type calcium channels. Neuron, 22(3), 549-58. [PubMed:10197534] [WorldCat] [DOI] - ↑
Zühlke, R.D., Pitt, G.S., Deisseroth, K., Tsien, R.W., & Reuter, H. (1999).
Calmodulin supports both inactivation and facilitation of L-type calcium channels. Nature, 399(6732), 159-62. [PubMed:10335846] [WorldCat] [DOI] - ↑
Lee, A., Wong, S.T., Gallagher, D., Li, B., Storm, D.R., Scheuer, T., & Catterall, W.A. (1999).
Ca2+/calmodulin binds to and modulates P/Q-type calcium channels. Nature, 399(6732), 155-9. [PubMed:10335845] [WorldCat] [DOI] - ↑
Maeda, N., Kawasaki, T., Nakade, S., Yokota, N., Taguchi, T., Kasai, M., & Mikoshiba, K. (1991).
Structural and functional characterization of inositol 1,4,5-trisphosphate receptor channel from mouse cerebellum. The Journal of biological chemistry, 266(2), 1109-16. [PubMed:1845986] [WorldCat] - ↑
Walsh, M.P., Dabrowska, R., Hinkins, S., & Hartshorne, D.J. (1982).
Calcium-independent myosin light chain kinase of smooth muscle. Preparation by limited chymotryptic digestion of the calcium ion dependent enzyme, purification, and characterization. Biochemistry, 21(8), 1919-25. [PubMed:6896283] [WorldCat] [DOI] - ↑
Blumenthal, D.K., Takio, K., Edelman, A.M., Charbonneau, H., Titani, K., Walsh, K.A., & Krebs, E.G. (1985).
Identification of the calmodulin-binding domain of skeletal muscle myosin light chain kinase. Proceedings of the National Academy of Sciences of the United States of America, 82(10), 3187-91. [PubMed:3858814] [PMC] [WorldCat] [DOI] - ↑
Lukas, T.J., Burgess, W.H., Prendergast, F.G., Lau, W., & Watterson, D.M. (1986).
Calmodulin binding domains: characterization of a phosphorylation and calmodulin binding site from myosin light chain kinase. Biochemistry, 25(6), 1458-64. [PubMed:3754463] [WorldCat] [DOI] - ↑
Foster, C., Van Fleet, M., & Marshak, A. (1986).
Tryptic digestion of myosin light chain kinase produces an inactive fragment that is activated on continued digestion. Archives of biochemistry and biophysics, 251(2), 616-23. [PubMed:3800388] [WorldCat] [DOI] - ↑
Kennedy, M.B., & Greengard, P. (1981).
Two calcium/calmodulin-dependent protein kinases, which are highly concentrated in brain, phosphorylate protein I at distinct sites. Proceedings of the National Academy of Sciences of the United States of America, 78(2), 1293-7. [PubMed:6785753] [PMC] [WorldCat] [DOI] - ↑
Schulman, H., & Greengard, P. (1978).
Stimulation of brain membrane protein phosphorylation by calcium and an endogenous heat-stable protein. Nature, 271(5644), 478-9. [PubMed:628428] [WorldCat] [DOI] - ↑
Yamauchi, T., & Fujisawa, H. (1980).
Evidence for three distinct forms of calmodulin-dependent protein kinases from rat brain. FEBS letters, 116(2), 141-4. [PubMed:7409141] [WorldCat] [DOI] - ↑
Ohmstede, C.A., Jensen, K.F., & Sahyoun, N.E. (1989).
Ca2+/calmodulin-dependent protein kinase enriched in cerebellar granule cells. Identification of a novel neuronal calmodulin-dependent protein kinase. The Journal of biological chemistry, 264(10), 5866-75. [PubMed:2538431] [WorldCat] - ↑
Tokumitsu, H., Brickey, D.A., Glod, J., Hidaka, H., Sikela, J., & Soderling, T.R. (1994).
Activation mechanisms for Ca2+/calmodulin-dependent protein kinase IV. Identification of a brain CaM-kinase IV kinase. The Journal of biological chemistry, 269(46), 28640-7. [PubMed:7961813] [WorldCat] - ↑
Haribabu, B., Hook, S.S., Selbert, M.A., Goldstein, E.G., Tomhave, E.D., Edelman, A.M., ..., & Means, A.R. (1995).
Human calcium-calmodulin dependent protein kinase I: cDNA cloning, domain structure and activation by phosphorylation at threonine-177 by calcium-calmodulin dependent protein kinase I kinase. The EMBO journal, 14(15), 3679-86. [PubMed:7641687] [PMC] [WorldCat] - ↑
Cohen, P., Burchell, A., Foulkes, J.G., Cohen, P.T., Vanaman, T.C., & Nairn, C. (1978).
Identification of the Ca2+-dependent modulator protein as the fourth subunit of rabbit skeletal muscle phosphorylase kinase. FEBS letters, 92(2), 287-93. [PubMed:212300] [WorldCat] [DOI] - ↑
Wang, J.H., & Desai, R. (1977).
Modulator binding protein. Bovine brain protein exhibiting the Ca2+-dependent association with the protein modulator of cyclic nucleotide phosphodiesterase. The Journal of biological chemistry, 252(12), 4175-84. [PubMed:193860] [WorldCat] - ↑
Klee, C.B., & Krinks, M.H. (1978).
Purification of cyclic 3',5'-nucleotide phosphodiesterase inhibitory protein by affinity chromatography on activator protein coupled to Sepharose. Biochemistry, 17(1), 120-6. [PubMed:201280] [WorldCat] [DOI] - ↑ 54.0 54.1
Lee, Y.C., & Wolff, J. (1984).
Calmodulin binds to both microtubule-associated protein 2 and tau proteins. The Journal of biological chemistry, 259(2), 1226-30. [PubMed:6420403] [WorldCat] - ↑
Gardner, K., & Bennett, V. (1986).
A new erythrocyte membrane-associated protein with calmodulin binding activity. Identification and purification. The Journal of biological chemistry, 261(3), 1339-48. [PubMed:3511042] [WorldCat] - ↑
Matsudaira, P.T., & Burgess, D.R. (1979).
Identification and organization of the components in the isolated microvillus cytoskeleton. The Journal of cell biology, 83(3), 667-73. [PubMed:574874] [PMC] [WorldCat] [DOI] - ↑
Carboni, J.M., Conzelman, K.A., Adams, R.A., Kaiser, D.A., Pollard, T.D., & Mooseker, M.S. (1988).
Structural and immunological characterization of the myosin-like 110-kD subunit of the intestinal microvillar 110K-calmodulin complex: evidence for discrete myosin head and calmodulin-binding domains. The Journal of cell biology, 107(5), 1749-57. [PubMed:2460467] [PMC] [WorldCat] [DOI] - ↑
Garcia, A., Coudrier, E., Carboni, J., Anderson, J., Vandekerkhove, J., Mooseker, M., ..., & Arpin, M. (1989).
Partial deduced sequence of the 110-kD-calmodulin complex of the avian intestinal microvillus shows that this mechanoenzyme is a member of the myosin I family. The Journal of cell biology, 109(6 Pt 1), 2895-903. [PubMed:2687288] [PMC] [WorldCat] [DOI] - ↑
Mooseker, M.S., & Coleman, T.R. (1989).
The 110-kD protein-calmodulin complex of the intestinal microvillus (brush border myosin I) is a mechanoenzyme. The Journal of cell biology, 108(6), 2395-400. [PubMed:2525564] [PMC] [WorldCat] [DOI] - ↑
Bredt, D.S., & Snyder, S.H. (1990).
Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proceedings of the National Academy of Sciences of the United States of America, 87(2), 682-5. [PubMed:1689048] [PMC] [WorldCat] [DOI] - ↑
Förstermann, U., Gorsky, L.D., Pollock, J.S., Ishii, K., Schmidt, H.H., Heller, M., & Murad, F. (1990).
Hormone-induced biosynthesis of endothelium-derived relaxing factor/nitric oxide-like material in N1E-115 neuroblastoma cells requires calcium and calmodulin. Molecular pharmacology, 38(1), 7-13. [PubMed:2370855] [WorldCat] - ↑
Nishida, E., Koyasu, S., Sakai, H., & Yahara, I. (1986).
Calmodulin-regulated binding of the 90-kDa heat shock protein to actin filaments. The Journal of biological chemistry, 261(34), 16033-6. [PubMed:3782106] [WorldCat] - ↑
Stevenson, M.A., & Calderwood, S.K. (1990).
Members of the 70-kilodalton heat shock protein family contain a highly conserved calmodulin-binding domain. Molecular and cellular biology, 10(3), 1234-8. [PubMed:2154682] [PMC] [WorldCat] [DOI] - ↑
Fink, C.C., Bayer, K.U., Myers, J.W., Ferrell, J.E., Schulman, H., & Meyer, T. (2003).
Selective regulation of neurite extension and synapse formation by the beta but not the alpha isoform of CaMKII. Neuron, 39(2), 283-97. [PubMed:12873385] [WorldCat] [DOI] - ↑
Takemoto-Kimura, S., Ageta-Ishihara, N., Nonaka, M., Adachi-Morishima, A., Mano, T., Okamura, M., ..., & Bito, H. (2007).
Regulation of dendritogenesis via a lipid-raft-associated Ca2+/calmodulin-dependent protein kinase CLICK-III/CaMKIgamma. Neuron, 54(5), 755-70. [PubMed:17553424] [WorldCat] [DOI] - ↑
Wen, Z., Guirland, C., Ming, G.L., & Zheng, J.Q. (2004).
A CaMKII/calcineurin switch controls the direction of Ca(2+)-dependent growth cone guidance. Neuron, 43(6), 835-46. [PubMed:15363394] [WorldCat] [DOI] - ↑
Ageta-Ishihara, N., Takemoto-Kimura, S., Nonaka, M., Adachi-Morishima, A., Suzuki, K., Kamijo, S., ..., & Bito, H. (2009).
Control of cortical axon elongation by a GABA-driven Ca2+/calmodulin-dependent protein kinase cascade. The Journal of neuroscience : the official journal of the Society for Neuroscience, 29(43), 13720-9. [PubMed:19864584] [PMC] [WorldCat] [DOI] - ↑
Tojima, T., Itofusa, R., & Kamiguchi, H. (2014).
Steering neuronal growth cones by shifting the imbalance between exocytosis and endocytosis. The Journal of neuroscience : the official journal of the Society for Neuroscience, 34(21), 7165-78. [PubMed:24849351] [PMC] [WorldCat] [DOI] - ↑
Saneyoshi, T., Wayman, G., Fortin, D., Davare, M., Hoshi, N., Nozaki, N., ..., & Soderling, T.R. (2008).
Activity-dependent synaptogenesis: regulation by a CaM-kinase kinase/CaM-kinase I/betaPIX signaling complex. Neuron, 57(1), 94-107. [PubMed:18184567] [PMC] [WorldCat] [DOI] - ↑
Hong, K., Nishiyama, M., Henley, J., Tessier-Lavigne, M., & Poo, M. (2000).
Calcium signalling in the guidance of nerve growth by netrin-1. Nature, 403(6765), 93-8. [PubMed:10638760] [WorldCat] [DOI] - ↑
Wang, G.X., & Poo, M.M. (2005).
Requirement of TRPC channels in netrin-1-induced chemotropic turning of nerve growth cones. Nature, 434(7035), 898-904. [PubMed:15758951] [WorldCat] [DOI] - ↑
Togashi, K., von Schimmelmann, M.J., Nishiyama, M., Lim, C.S., Yoshida, N., Yun, B., ..., & Hong, K. (2008).
Cyclic GMP-gated CNG channels function in Sema3A-induced growth cone repulsion. Neuron, 58(5), 694-707. [PubMed:18549782] [WorldCat] [DOI] - ↑
Nishiyama, M., von Schimmelmann, M.J., Togashi, K., Findley, W.M., & Hong, K. (2008).
Membrane potential shifts caused by diffusible guidance signals direct growth-cone turning. Nature neuroscience, 11(7), 762-71. [PubMed:18536712] [WorldCat] [DOI] - ↑
Hudmon, A., & Schulman, H. (2002).
Neuronal CA2+/calmodulin-dependent protein kinase II: the role of structure and autoregulation in cellular function. Annual review of biochemistry, 71, 473-510. [PubMed:12045104] [WorldCat] [DOI] - ↑
Woodgett, J.R., Davison, M.T., & Cohen, P. (1983).
The calmodulin-dependent glycogen synthase kinase from rabbit skeletal muscle. Purification, subunit structure and substrate specificity. European journal of biochemistry, 136(3), 481-7. [PubMed:6315430] [WorldCat] [DOI] - ↑
Chao, L.H., Stratton, M.M., Lee, I.H., Rosenberg, O.S., Levitz, J., Mandell, D.J., ..., & Kuriyan, J. (2011).
A mechanism for tunable autoinhibition in the structure of a human Ca2+/calmodulin- dependent kinase II holoenzyme. Cell, 146(5), 732-45. [PubMed:21884935] [PMC] [WorldCat] [DOI] - ↑
Meyer, T., Hanson, P.I., Stryer, L., & Schulman, H. (1992).
Calmodulin trapping by calcium-calmodulin-dependent protein kinase. Science (New York, N.Y.), 256(5060), 1199-202. [PubMed:1317063] [WorldCat] [DOI] - ↑
Miller, S.G., & Kennedy, M.B. (1986).
Regulation of brain type II Ca2+/calmodulin-dependent protein kinase by autophosphorylation: a Ca2+-triggered molecular switch. Cell, 44(6), 861-70. [PubMed:3006921] [WorldCat] [DOI] - ↑
Lou, L.L., Lloyd, S.J., & Schulman, H. (1986).
Activation of the multifunctional Ca2+/calmodulin-dependent protein kinase by autophosphorylation: ATP modulates production of an autonomous enzyme. Proceedings of the National Academy of Sciences of the United States of America, 83(24), 9497-501. [PubMed:3467320] [PMC] [WorldCat] [DOI] - ↑
Malinow, R., Madison, D.V., & Tsien, R.W. (1988).
Persistent protein kinase activity underlying long-term potentiation. Nature, 335(6193), 820-4. [PubMed:2847049] [WorldCat] [DOI] - ↑
Malenka, R.C., Kauer, J.A., Perkel, D.J., Mauk, M.D., Kelly, P.T., Nicoll, R.A., & Waxham, M.N. (1989).
An essential role for postsynaptic calmodulin and protein kinase activity in long-term potentiation. Nature, 340(6234), 554-7. [PubMed:2549423] [WorldCat] [DOI] - ↑
Silva, A.J., Stevens, C.F., Tonegawa, S., & Wang, Y. (1992).
Deficient hippocampal long-term potentiation in alpha-calcium-calmodulin kinase II mutant mice. Science (New York, N.Y.), 257(5067), 201-6. [PubMed:1378648] [WorldCat] [DOI] - ↑
Silva, A.J., Paylor, R., Wehner, J.M., & Tonegawa, S. (1992).
Impaired spatial learning in alpha-calcium-calmodulin kinase II mutant mice. Science (New York, N.Y.), 257(5067), 206-11. [PubMed:1321493] [WorldCat] [DOI] - ↑
Giese, K.P., Fedorov, N.B., Filipkowski, R.K., & Silva, A.J. (1998).
Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science (New York, N.Y.), 279(5352), 870-3. [PubMed:9452388] [WorldCat] [DOI] - ↑
Mulkey, R.M., Endo, S., Shenolikar, S., & Malenka, R.C. (1994).
Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature, 369(6480), 486-8. [PubMed:7515479] [WorldCat] [DOI] - ↑
Zhuo, M., Zhang, W., Son, H., Mansuy, I., Sobel, R.A., Seidman, J., & Kandel, E.R. (1999).
A selective role of calcineurin aalpha in synaptic depotentiation in hippocampus. Proceedings of the National Academy of Sciences of the United States of America, 96(8), 4650-5. [PubMed:10200317] [PMC] [WorldCat] [DOI] - ↑
Wong, S.T., Athos, J., Figueroa, X.A., Pineda, V.V., Schaefer, M.L., Chavkin, C.C., ..., & Storm, D.R. (1999).
Calcium-stimulated adenylyl cyclase activity is critical for hippocampus-dependent long-term memory and late phase LTP. Neuron, 23(4), 787-98. [PubMed:10482244] [WorldCat] [DOI] - ↑
Zeng, H., Chattarji, S., Barbarosie, M., Rondi-Reig, L., Philpot, B.D., Miyakawa, T., ..., & Tonegawa, S. (2001).
Forebrain-specific calcineurin knockout selectively impairs bidirectional synaptic plasticity and working/episodic-like memory. Cell, 107(5), 617-29. [PubMed:11733061] [WorldCat] [DOI] - ↑
Matsuzaki, M., Honkura, N., Ellis-Davies, G.C., & Kasai, H. (2004).
Structural basis of long-term potentiation in single dendritic spines. Nature, 429(6993), 761-6. [PubMed:15190253] [PMC] [WorldCat] [DOI] - ↑
Zhou, Q., Homma, K.J., & Poo, M.M. (2004).
Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron, 44(5), 749-57. [PubMed:15572107] [WorldCat] [DOI] - ↑
Oh, W.C., Hill, T.C., & Zito, K. (2013).
Synapse-specific and size-dependent mechanisms of spine structural plasticity accompanying synaptic weakening. Proceedings of the National Academy of Sciences of the United States of America, 110(4), E305-12. [PubMed:23269840] [PMC] [WorldCat] [DOI] - ↑
Honkura, N., Matsuzaki, M., Noguchi, J., Ellis-Davies, G.C., & Kasai, H. (2008).
The subspine organization of actin fibers regulates the structure and plasticity of dendritic spines. Neuron, 57(5), 719-29. [PubMed:18341992] [WorldCat] [DOI] - ↑
Okamoto, K., Narayanan, R., Lee, S.H., Murata, K., & Hayashi, Y. (2007).
The role of CaMKII as an F-actin-bundling protein crucial for maintenance of dendritic spine structure. Proceedings of the National Academy of Sciences of the United States of America, 104(15), 6418-23. [PubMed:17404223] [PMC] [WorldCat] [DOI] - ↑
Nishiyama, J., & Yasuda, R. (2015).
Biochemical Computation for Spine Structural Plasticity. Neuron, 87(1), 63-75. [PubMed:26139370] [PMC] [WorldCat] [DOI] - ↑
Lee, S.J., Escobedo-Lozoya, Y., Szatmari, E.M., & Yasuda, R. (2009).
Activation of CaMKII in single dendritic spines during long-term potentiation. Nature, 458(7236), 299-304. [PubMed:19295602] [PMC] [WorldCat] [DOI] - ↑
Fujii, H., Inoue, M., Okuno, H., Sano, Y., Takemoto-Kimura, S., Kitamura, K., ..., & Bito, H. (2013).
Nonlinear decoding and asymmetric representation of neuronal input information by CaMKIIα and calcineurin. Cell reports, 3(4), 978-87. [PubMed:23602566] [WorldCat] [DOI] - ↑
Bito, H., Deisseroth, K., & Tsien, R.W. (1996).
CREB phosphorylation and dephosphorylation: a Ca(2+)- and stimulus duration-dependent switch for hippocampal gene expression. Cell, 87(7), 1203-14. [PubMed:8980227] [WorldCat] [DOI] - ↑
Kawashima, T., Okuno, H., Nonaka, M., Adachi-Morishima, A., Kyo, N., Okamura, M., ..., & Bito, H. (2009).
Synaptic activity-responsive element in the Arc/Arg3.1 promoter essential for synapse-to-nucleus signaling in activated neurons. Proceedings of the National Academy of Sciences of the United States of America, 106(1), 316-21. [PubMed:19116276] [PMC] [WorldCat] [DOI] - ↑
Nonaka, M., Kim, R., Fukushima, H., Sasaki, K., Suzuki, K., Okamura, M., ..., & Bito, H. (2014).
Region-specific activation of CRTC1-CREB signaling mediates long-term fear memory. Neuron, 84(1), 92-106. [PubMed:25277455] [WorldCat] [DOI] - ↑
Bhalla, U.S. (2002).
Biochemical signaling networks decode temporal patterns of synaptic input. Journal of computational neuroscience, 13(1), 49-62. [PubMed:12154335] [WorldCat] - ↑
Fujii, H., Inoue, M., Okuno, H., Sano, Y., Takemoto-Kimura, S., Kitamura, K., ..., & Bito, H. (2013).
Nonlinear decoding and asymmetric representation of neuronal input information by CaMKIIα and calcineurin. Cell reports, 3(4), 978-87. [PubMed:23602566] [WorldCat] [DOI] - ↑ B Weiss, R. Fertel, R Figlin, and P Uzunov
Selective alteration of the activity of the multiple forms of adenosine 3', 5'-monophosphate phosphodiesterase of rat cerebrum
Mol. Pharmacol. 10, 615-625:1974 - ↑
Honda, F., & Imamura, H. (1968).
Inhibition of cyclic 3',5'-nucleotide phosphodiesterase by phenothiazine and reserpine derivatives. Biochimica et biophysica acta, 161(1), 267-9. [PubMed:4298921] [WorldCat] [DOI] - ↑
Tanaka, T., & Hidaka, H. (1980).
Hydrophobic regions function in calmodulin-enzyme(s) interactions. The Journal of biological chemistry, 255(23), 11078-80. [PubMed:6254958] [WorldCat] - ↑ H Van Belle
R 24 571: A potent inhibitor of calmodulin-activated enzymes.
Cell Calcium 2, 483-494:1981 - ↑
Martínez-Luis, S., Pérez-Vásquez, A., & Mata, R. (2007).
Natural products with calmodulin inhibitor properties. Phytochemistry, 68(14), 1882-903. [PubMed:17400264] [WorldCat] [DOI] - ↑
Mata, R., Figueroa, M., González-Andrade, M., Rivera-Chávez, J.A., Madariaga-Mazón, A., & Del Valle, P. (2015).
Calmodulin inhibitors from natural sources: an update. Journal of natural products, 78(3), 576-86. [PubMed:25536331] [WorldCat] [DOI] - ↑ 108.0 108.1
Nyegaard, M., Overgaard, M.T., Søndergaard, M.T., Vranas, M., Behr, E.R., Hildebrandt, L.L., ..., & Børglum, A.D. (2012).
Mutations in calmodulin cause ventricular tachycardia and sudden cardiac death. American journal of human genetics, 91(4), 703-12. [PubMed:23040497] [PMC] [WorldCat] [DOI] - ↑ 109.0 109.1 109.2
Crotti, L., Johnson, C.N., Graf, E., De Ferrari, G.M., Cuneo, B.F., Ovadia, M., ..., & George, A.L. (2013).
Calmodulin mutations associated with recurrent cardiac arrest in infants. Circulation, 127(9), 1009-17. [PubMed:23388215] [PMC] [WorldCat] [DOI] - ↑ 110.0 110.1 110.2 110.3 110.4
Makita, N., Yagihara, N., Crotti, L., Johnson, C.N., Beckmann, B.M., Roh, M.S., ..., & George, A.L. (2014).
Novel calmodulin mutations associated with congenital arrhythmia susceptibility. Circulation. Cardiovascular genetics, 7(4), 466-74. [PubMed:24917665] [PMC] [WorldCat] [DOI] - ↑
Reed, G.J., Boczek, N.J., Etheridge, S.P., & Ackerman, M.J. (2015).
CALM3 mutation associated with long QT syndrome. Heart rhythm, 12(2), 419-22. [PubMed:25460178] [PMC] [WorldCat] [DOI] - ↑
Marsman, R.F., Barc, J., Beekman, L., Alders, M., Dooijes, D., van den Wijngaard, A., ..., & Bezzina, C.R. (2014).
A mutation in CALM1 encoding calmodulin in familial idiopathic ventricular fibrillation in childhood and adolescence. Journal of the American College of Cardiology, 63(3), 259-66. [PubMed:24076290] [WorldCat] [DOI] - ↑ CALM1 COSMIC database
- ↑ CALM2 COSMIC database
- ↑ CALM3 COSMIC database
- ↑
Romoser, V.A., Hinkle, P.M., & Persechini, A. (1997).
Detection in living cells of Ca2+-dependent changes in the fluorescence emission of an indicator composed of two green fluorescent protein variants linked by a calmodulin-binding sequence. A new class of fluorescent indicators. The Journal of biological chemistry, 272(20), 13270-4. [PubMed:9148946] [WorldCat] [DOI] - ↑
Miyawaki, A., Llopis, J., Heim, R., McCaffery, J.M., Adams, J.A., Ikura, M., & Tsien, R.Y. (1997).
Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature, 388(6645), 882-7. [PubMed:9278050] [WorldCat] [DOI] - ↑
Nakai, J., Ohkura, M., & Imoto, K. (2001).
A high signal-to-noise Ca(2+) probe composed of a single green fluorescent protein. Nature biotechnology, 19(2), 137-41. [PubMed:11175727] [WorldCat] [DOI] - ↑
Nagai, T., Sawano, A., Park, E.S., & Miyawaki, A. (2001).
Circularly permuted green fluorescent proteins engineered to sense Ca2+. Proceedings of the National Academy of Sciences of the United States of America, 98(6), 3197-202. [PubMed:11248055] [PMC] [WorldCat] [DOI] - ↑
Nagai, T., Yamada, S., Tominaga, T., Ichikawa, M., & Miyawaki, A. (2004).
Expanded dynamic range of fluorescent indicators for Ca(2+) by circularly permuted yellow fluorescent proteins. Proceedings of the National Academy of Sciences of the United States of America, 101(29), 10554-9. [PubMed:15247428] [PMC] [WorldCat] [DOI] - ↑
Palmer, A.E., Giacomello, M., Kortemme, T., Hires, S.A., Lev-Ram, V., Baker, D., & Tsien, R.Y. (2006).
Ca2+ indicators based on computationally redesigned calmodulin-peptide pairs. Chemistry & biology, 13(5), 521-30. [PubMed:16720273] [WorldCat] [DOI] - ↑
Wallace, D.J., Meyer zum Alten Borgloh, S., Astori, S., Yang, Y., Bausen, M., Kügler, S., ..., & Hasan, M.T. (2008).
Single-spike detection in vitro and in vivo with a genetic Ca2+ sensor. Nature methods, 5(9), 797-804. [PubMed:19160514] [WorldCat] [DOI] - ↑
Mank, M., Santos, A.F., Direnberger, S., Mrsic-Flogel, T.D., Hofer, S.B., Stein, V., ..., & Griesbeck, O. (2008).
A genetically encoded calcium indicator for chronic in vivo two-photon imaging. Nature methods, 5(9), 805-11. [PubMed:19160515] [WorldCat] [DOI] - ↑
Tian, L., Hires, S.A., Mao, T., Huber, D., Chiappe, M.E., Chalasani, S.H., ..., & Looger, L.L. (2009).
Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nature methods, 6(12), 875-81. [PubMed:19898485] [PMC] [WorldCat] [DOI] - ↑
Zhao, Y., Araki, S., Wu, J., Teramoto, T., Chang, Y.F., Nakano, M., ..., & Campbell, R.E. (2011).
An expanded palette of genetically encoded Ca²⁺ indicators. Science (New York, N.Y.), 333(6051), 1888-91. [PubMed:21903779] [PMC] [WorldCat] [DOI] - ↑
Chen, T.W., Wardill, T.J., Sun, Y., Pulver, S.R., Renninger, S.L., Baohan, A., ..., & Kim, D.S. (2013).
Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature, 499(7458), 295-300. [PubMed:23868258] [PMC] [WorldCat] [DOI] - ↑
Thestrup, T., Litzlbauer, J., Bartholomäus, I., Mues, M., Russo, L., Dana, H., ..., & Griesbeck, O. (2014).
Optimized ratiometric calcium sensors for functional in vivo imaging of neurons and T lymphocytes. Nature methods, 11(2), 175-82. [PubMed:24390440] [WorldCat] [DOI] - ↑
Inoue, M., Takeuchi, A., Horigane, S., Ohkura, M., Gengyo-Ando, K., Fujii, H., ..., & Bito, H. (2015).
Rational design of a high-affinity, fast, red calcium indicator R-CaMP2. Nature methods, 12(1), 64-70. [PubMed:25419959] [WorldCat] [DOI] - ↑
Fosque, B.F., Sun, Y., Dana, H., Yang, C.T., Ohyama, T., Tadross, M.R., ..., & Schreiter, E.R. (2015).
Neural circuits. Labeling of active neural circuits in vivo with designed calcium integrators. Science (New York, N.Y.), 347(6223), 755-60. [PubMed:25678659] [WorldCat] [DOI]